Article Text
Abstract
Asthma severity and control can be measured both subjectively and objectively. Traditionally asthma treatments have been individualised using symptoms and spirometry/peak flow. Increasingly treatment tailored in accordance with inflammatory markers (sputum eosinophil counts or fractional exhaled nitric oxide (FeNO) data) is advocated as an alternative strategy. The objective of this review was to evaluate the efficacy of tailoring asthma interventions based on inflammatory markers (sputum analysis and FeNO) in comparison with clinical symptoms (with or without spirometry/peak flow) for asthma-related outcomes in children and adults. Cochrane Airways Group Specialised Register of Trials, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE and reference lists of articles were searched. The last searches were in February 2009. All randomised controlled comparisons of adjustment of asthma treatment based on sputum analysis or FeNO compared with traditional methods (primarily clinical symptoms and spirometry/peak flow) were selected. Results of searches were reviewed against predetermined criteria for inclusion. Relevant studies were selected, assessed and data extracted independently by at least two people. The trial authors were contacted for further information. Data were analysed as ‘intervention received’ and sensitivity analyses performed. Six (2 adults and 4 children/adolescent) studies utilising FeNO and three adult studies utilising sputum eosinophils were included. These studies had a degree of clinical heterogeneity including definition of asthma exacerbations, duration of study and variations in cut-off levels for percentage of sputum eosinophils and FeNO to alter management in each study. Adults who had treatment adjusted according to sputum eosinophils had a reduced number of exacerbations compared with the control group (52 vs 77 patients with ≥1 exacerbation in the study period; p=0.0006). There was no significant difference in exacerbations between groups for FeNO compared with controls. The daily dose of inhaled corticosteroids at the end of the study was decreased in adults whose treatment was based on FeNO in comparison with the control group (mean difference −450.03 μg, 95% CI −676.73 to −223.34; p<0.0001). However, children who had treatment adjusted according to FeNO had an increase in their mean daily dose of inhaled corticosteroids (mean difference 140.18 μg, 95% CI 28.94 to 251.42; p=0.014). It was concluded that tailoring of asthma treatment based on sputum eosinophils is effective in decreasing asthma exacerbations. However, tailoring of asthma treatment based on FeNO levels has not been shown to be effective in improving asthma outcomes in children and adults. At present, there is insufficient justification to advocate the routine use of either sputum analysis (due to technical expertise required) or FeNO in everyday clinical practice.
- Asthma
- inflammatory markers
- exhaled nitric oxide
- systematic review
- exhaled airway markers
Statistics from Altmetric.com
Key messages
What is the key question?
What is the overall outcome of trials assessing the use of the sputum eosinophil counts and exhaled nitric oxide to tailor asthma treatment?
What is the key point?
Treatment tailored using the sputum eosinophil count results in fewer asthma attacks than traditional management in adults with severe asthma; the overall findings with exhaled nitric oxide are negative but they are difficult to interpret because of differences in methodology.
Why read on?
There has been considerable interest in inflammometry in asthma management and the benefits of sputum guided management in severe asthma are marked.
Introduction
Monitoring tools to assist in improving asthma control and prevention of exacerbations are two key elements in asthma guidelines.1–3 There is no single outcome measure that can adequately assess asthma control.4 Subjective measures usually involve a series of questions used for clinical assessment, diary cards and quality of life (QoL) questionnaires. Traditional objective methods used to monitor (but not control) asthma include spirometry/peak flow and degree of airway hyper-responsiveness (AHR).5 Newer methods include measurement of airway inflammation such as airway cellularity in induced sputum or fractional exhaled nitric oxide (FeNO).
The inflammation in airways of people with asthma can be predominantly eosinophilic or non-eosinophilic (including neutrophilic).6 Irrespective of the type of airway inflammation, inhaled corticosteroids (ICS) remain the major preventer treatment to control asthma symptoms in those with asthma, other than children with mild intermittent asthma.3 However, ICS are more effective in reducing symptoms in patients with eosinophilic inflammation than those with neutrophilic inflammation.7 Thus investigations that provide objective data on eosinophilic inflammation may be helpful in reducing exacerbations and improve asthma control. Current available techniques for clinical use are assessment of sputum cellularity and FeNO.8
A systematic review evaluating the efficacy of tailoring asthma interventions based on utilising sputum eosinophils or FeNO in comparison with current strategy (reliance on clinical symptoms with or without spirometry/peak flow) will be useful to guide clinical practice. Here we combine two Cochrane reviews9 10 that address this question. The objective of this systematic review is to evaluate the efficacy of tailoring asthma interventions based on FeNO or sputum eosinophils in comparison with controls (clinical symptoms with or without spirometry/peak flow) for asthma-related outcomes in children and adults.
Methods
Methods of the analysis and inclusion criteria were specified in advance and documented in protocols that are available alongside the original versions of these reviews in The Cochrane Library.
Eligibility, information sources, search strategy and study selection
We used the PRISMA guidelines,11 Cochrane collaboration methodology and software (RevMan5). We searched the Cochrane Airways Group specialised register for eligible randomised controlled trials that compared adjustment of asthma medications based on sputum eosinophils or FeNO levels in comparison with clinical symptoms (with or without spirometry/peak flow) using keywords in electronic sources (Cochrane Airways Group Specialised Register of Trials, the Cochrane Central Register of Controlled Trials (CENTRAL), Medline, EMBASE) and hand searching of references as outlined in the reviews.9 10 The latest searches were performed in February 2009. Trials that included the use of other interventions were included if all participants had equal access to such interventions.
Participant inclusion criteria were children and adults with 'classical asthma'. Exclusion criteria were: eosinophilic bronchitis, asthma related to an underlying lung disease such as bronchiectasis and chronic obstructive airway disease, or diagnostic categories such as 'cough variant asthma' and 'wheezy bronchitis' where controversies exist.
Data items
From the title, abstract or descriptors, the literature search was reviewed independently in triplicate to identify potentially relevant trials for full review. Searches of bibliographies and texts were conducted to identify additional studies. From the full text using specific criteria, two reviewers independently selected trials for inclusion. There was no disagreement, although it was planned that disagreement would have been resolved by third-party adjudication. We extracted information from each trial on (1) study characteristics, (2) intervention type and (3) outcomes, as described in our Cochrane reviews.9 10
Risk of bias
Risk of bias for each study was assessed using the tool available in the RevMan software. Six components were assessed: (1) adequate sequence generation; (2) allocation concealment; (3) blinding; (4) incomplete outcome data addressed; (5) free of selective reporting; and (6) free of other bias. Studies included in the review underwent quality assessment and were entered into a ‘risk of bias’ table.
Summary (outcome) measures
Primary outcomes were the number of participants who had asthma exacerbations during follow-up. Secondary outcomes were mean difference in asthma-related outcome measures, number of participants experiencing adverse effects of the interventions and number of participants experiencing complications such as requirement for medication change. The proportions of participants and the mean clinical improvement were determined using the following hierarchy of assessment measures (ie, where two or more assessment measures are reported in the same study, the outcome measure that is listed first in the hierarchy was used);
Hospitalisation, acute presentations to an emergency facility for asthma.
Rescue courses of oral corticosteroids.
Symptomatic (QoL, Likert scale, asthma diary, visual analogue scale)—assessed by the patient (adult or child).
Symptomatic (QoL, Likert scale, asthma diary, visual analogue scale)—assessed by the parents/carers.
Symptomatic (Likert scale, visual analogue scale)—assessed by clinicians.
Indices of spirometry, peak flow, AHR.
β-Agonist used.
In addition, dose of ICS used was also analysed as a post hoc analysis.
Methods of analysis
The results from studies that met the inclusion criteria and reported any of the outcomes of interest were included in the subsequent meta-analyses. All data were double entered (HP and AC) and triple checked (CC). For the dichotomous outcome variables of each individual study, relative and absolute risk reductions were calculated using a modified intention-to-treat analysis when the outcome event is a beneficial event. When the event is non-beneficial (such as exacerbation), ‘treatment received’ analysis was utilised. The summary weighted RR and 95% CI (fixed effect model) were calculated (Cochrane statistical package, RevMan 5.0). For rate ratios of common events whereby one subject may have more than one event, generic inverse variance (GIV) was utilised. The rate ratios were taken from the published papers and the standard errors were calculated from CIs or p values published in the papers. Number needed to treat (NNT) was calculated from the pooled OR and its 95% CI applied to a specified baseline risk using an online calculator.12 If studies reported outcomes using different measurement scales, the standardised mean difference was estimated. Any heterogeneity between the study results was described and tested to see if it reached statistical significance using a χ2 test. The 95% CI estimated using a random effects model was included whenever there were concerns about statistical heterogeneity. Heterogeneity was considered significant when the p value is <0.10.13 An a priori subgroup analysis was planned for adults versus children.
Results
Study selection and study characteristics
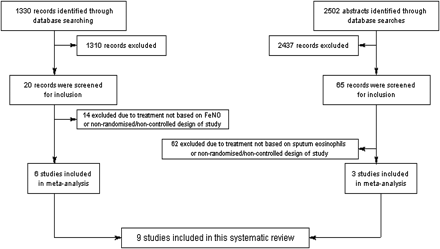
The searches identified 1330 FeNO-based studies and 2502 sputum studies (figure 1). After screening 20 and 65 papers, respectively, 6 and 3, respectively, fulfilled the inclusion criteria (figure 1) for the interventions. The nine studies (3 adult studies utilising sputum eosinophils, 6 studies utilising FeNO—2 adults, 4 children) involved 1299 participants, with 1231 completing.
PRISMA flow chart.
Of the nine studies included (table 1), six were unicentre studies14–19 and three were multicentred.20–22 Four studies were in children or adolescents,16 17 20 21 four with adult patients19 and one combining adolescents and adults.18 We classified studies into children/adolescent studies based on the mean age reported as opposed to the entry criteria. Four studies were double blind, parallel groups17 20 whereas five were single blind, parallel groups.16 18 19 21 All nine papers were published in English.
Characteristics of included studies
There was a degree a clinical heterogeneity between studies as summarised in table 1. Most variation related to the definition of an asthma exacerbation and the cut-off utilised for adjusting treatments. Although asthma exacerbations were an outcome measure in all papers, they differed in how they were defined, ranging from unscheduled emergency visits21 20 to defining an exacerbation using diary card data.18 Although there was variation in how exacerbations were defined, all included studies uniformly managed exacerbations with rescue oral steroids. Algorithms for adjustment of medications differed between studies and the cut-off values to step-up and step-down also varied across the FeNO studies (range from 2016 20 21 to 3518), and the sputum eosinophil percentages (range from 222 to 814).
Outcomes and synthesis of results
Primary (Exacerbations)
In FeNO-based adult studies (figure 2), the number of participants with exacerbations in the group with treatment adjusted according to FeNO was similar to the control group; 26 with exacerbations vs 30, respectively (p=0.763), OR 0.85 (95% CI 0.30 to 2.43). The number of children who had exacerbations in the FeNO-based group was not significantly different in the control group (102 vs 118, respectively, p=0.062), OR 0.75 (95% CI 0.55 to 1.01) (figure 2).
Number of subjects who had ≥1 exacerbation over the study period (fractional exhaled nitric oxide (FeNO)).
In contrast, in the sputum-based meta-analysis (figure 3) significantly fewer adults in the group that utilised sputum eosinophil count had asthma exacerbations compared with the control group (52 vs 77; p=0.0006), OR 0.36 (95% CI 0.20 to 0.64). NNT for benefit was 6 (95% CI 4–32) over 52 weeks.
Number of subject who had ≥1 exacerbation over the study period (sputum eosinophils (SpEos)).
Secondary outcomes
ICS dose
For FeNO-based studies, meta-analysis of adult studies was opposite to that of paediatric studies (figure 4). Adults who had treatment adjusted according to FeNO had a significantly lower dose of ICS at the end of the study period (figure 4) than those in the control group (mean difference between groups was −450.03 μg budesonide equivalent; 95% CI −676.73 to −223.34; p<0.0001). However, Shaw19 also reported an 11% increase in the total amount of ICS used during the study (95% CI −15% to 37%). In paediatric studies, the group who had treatment adjusted according to FeNO (figure 4) had significantly higher doses of ICS at the end of the study compared with the control group (mean difference 140.18, 95% CI 28.94 to 251.42; p=0.014).
Inhaled corticosteroid dose at final visit (fractional exhaled nitric oxide (FeNO)).
All three studies that utilised sputum eosinophils to adjust treatment reported no differences in doses of ICS used between groups (figure 5). The SDs for the groups were not available in Jayaram's paper and were estimated based on the data from Green's paper. Mean dose of ICS per person per day between groups was non-significant; weighted mean difference was 78.99, 95% CI −90.13 to 248.11; p=0.157.
Mean dose of inhaled corticosteroid per person per day (sputum eosinophils (SpEos)).
Symptom scores
Symptom scores did not differ between groups for FeNO-based studies in both adults and children (figure 6). In adults, the mean difference was –0.10, 95% CI −0.33 to 0.12; p=0.372. In children, the mean difference was 0.13, 95% CI −0.32 to 0.57; p=0.577. For the sputum-based studies, the two studies that reported on symptom scores also described no difference in symptoms scores between groups.14 15
Symptom score (fractional exhaled nitric oxide (FeNO)).
Sensitivity analyses
There were insufficient data reported from the individual studies to include other secondary outcomes (forced expiratory volume in 1 s (FEV1). AHR, rescue β-agonist use, QoL) for meta-analysis. FEV1 was an outcome in all nine studies; eight studies14 15 18 19 21 described no difference between the participants who had treatment adjusted to inflammatory markers in comparison with the control group.
Results from the sensitivity analyses did not alter direction or non-significance of primary outcomes (exacerbations) but changed the final ICS dose in the paediatric studies from favouring controls to a non-significant difference between groups (see supplementary file online).
Risk of bias in individual studies
The risk of bias table (table 2) shows that four studies15 17 19 22 were considered moderate to high quality, but in all studies there were insufficient details about either allocation concealment and/or adequacy of blinding. One study14 was open labelled while another21 was single blinded.
Risk of bias summary of included studies
For the FeNO-based papers, the quality of evidence using the GRADE approach surmises that of the four outcomes assessed, three were of moderate quality and one (ICS dose in children) was low quality due to one study21 being single blinded and a high final daily dose of ICS in another study20 (table 3). For sputum-based studies, GRADE assessment shows that the quality of both outcomes was low (exacerbation) and very low (ICS dose) due to the lack of blinding in one study14 and the high daily doses of ICS at the end of the study in two studies14 15 (table 4).
Grade assessment of FeNO-based papers
Grade assessment of sputum eosinophil-based papers
Discussion
In this meta-analysis, we combined data from our Cochrane reviews9 10 that evaluated the efficacy of tailoring asthma interventions based on FeNO or sputum eosinophils in comparison with controls (clinical symptoms with or without spirometry/peak flow) for asthma-related outcomes in children and adults. Based on nine studies in 1299 adults and children (1231 completed), we found that the number of adults who had an exacerbation (as defined by the author) was significantly lower in the group in which ICS was tailored based on sputum eosinophilia compared with the control group (ie, managed with the usual traditional methods, based primarily on clinical symptoms). In contrast there was no significant difference between groups when ICS was tailored based on FeNO. In children/adolescents there was a non-significant trend favouring the FeNO strategy in a number of participants with one or more exacerbations, but this was at the expense of higher levels of ICS. In adults, the FeNO-based strategy enabled a reduction in the final (but not the overall) daily dose of ICS. For both FeNO- and sputum-based strategies, there was no difference between groups for all secondary outcomes (FEV1, symptom scores, AHR and β2-agonist use).
Tailoring medications based on FeNO has been advocated in an editorial23 and is now relatively widely used in some countries where a rebate for its use is available. This meta-analysis has shown that the benefits of utilising an FeNO-based strategy (as opposed to a standard strategy based on clinical symptoms and simple tests such as FEV1) is at best modest and could potentially be harmful with increased ICS use in children. There was no significant difference between the two strategies in both adult and paediatric studies in the primary outcome of exacerbation when utilising FeNO. The only significant beneficial difference found between groups was the final daily dose of ICS in adults. However, this finding is limited as this was a post hoc analysis.
The primary outcome chosen was exacerbation, an important outcome as this affects the patient's QoL and the extent to which the patient can carry out their activities of daily life.4 Arguably this is the most important outcome in studies on efficacy of interventions for asthma control. Our meta-analysis has shown that in contrast to the non-beneficial effect of FeNO on rate of exacerbation, tailoring treatment based on sputum eosinophils decreased the number of exacerbations experienced by this group of adults.
In contrast to the favourable data in the outcome of exacerbations that support the use of sputum to guide asthma treatments in adults, there was no significant difference between the groups for both sputum- and FeNO-based strategies in other asthma outcomes (FEV1, QoL and β2-agonist use). While exacerbations are an important outcome, arguably subjective measures of asthma control are also important. Thus, although our findings demonstrate that monitoring airway inflammation through eosinophils in induced sputum is useful in reducing exacerbations, it is debatable whether it should be universally advocated. Furthermore, sputum analysis is restricted to laboratories with specific expertise in inducing and analysing sputum. Obtaining and analysing sputum is relatively time consuming (when compared with FeNO) and is not always successful, particularly in young children. Nevertheless, use of sputum induction to guide asthma treatment is most likely to be beneficial in adults with severe asthma and those with frequent exacerbations.
The FeNO-based studies need to be considered in light of several issues. First, none of the six included studies utilising FeNO considered presence or severity of atopy in their algorithm of management, although some but not all subjects were atopic. Raised FeNO in children has been associated with atopy with or without respiratory symptoms.24 Shaw and colleagues19 reported that some of their participants were atopic (62% in the FeNO group, 70% in the control group). Smith et al18 did not describe whether their subjects were atopic or not. ‘Atopic asthma' was an inclusion criterion for Pijnenburg et al25 as defined as RAST (radioallergosorbent test) class 2 or higher for at least one airborne allergen ever. Similarly, all children in the study of Fritsch et al16 had an inclusion criterion of positive skin prick test or RAST.
Secondly, the cut-offs of FeNO utilised for stepping up or down treatment differed between studies (range 15–30 ppb). The subjects of the study of Pijnenburg et al17 (paediatric study) had the highest mean daily dose of ICS and subjects in this study also had quite high FeNO at the final visit (∼25.5 pbb in the FeNO group, 36.7 in the controls). Disconcertingly, use of the FeNO strategy did not result in a lower FeNO level at the end of the trial. Smith et al18 mentioned that their 15 ppb threshold is equivalent to 35 ppb at a slower 50 ml/s flow rate. There is no evidence-based algorithm to adjust treatment in relation to FeNO levels (or indeed to sputum eosinophils levels). There are differences in guidelines (such as GINA1, BTS2, NAC3) with respect to when and how to step-up and step-down asthma treatments. Arguably the algorithm should provide a result sufficiently different from clinical decision making in order for there to be any benefit.26
The difference in results of using sputum eosinophils (beneficial for exacerbations) versus FeNO (not beneficial) is likely to be because FeNO levels do not necessarily reflect sputum eosinophil density, particularly in non-steroid-naïve patients.27 28 Also, consideration of cost is important for the universal use of FeNO in health systems. FeNO measurements require a nitric oxide analyser that needs maintenance and/or calibration. Nitric oxide analysers are relatively expensive, and adding FeNO as a monitoring tool adds not only cost but also another layer of complexity in asthma care. Analysers were only approved by the US Food and Drug Administration for clinical monitoring of anti-inflammatory treatment in 2003.29 As reported in the risk of bias table (table 2), accurate FeNO measurements at each visit could not be obtained, due either to a faulty analyser21 or to technical issues.16 Also, many aspects need to be considered when analysing FeNO; this includes the timing of spirometry (transiently reduces FeNO), food and beverage, circadian rhythm, smoking history, ambient nitric oxide and exercise.29
Limitations of the review
This systematic review is limited to nine studies with 1231 subjects completing the trials. While the studies share some common issues, there are also significant differences, notably the definition of asthma exacerbation, how the decision to prescribe oral steroids was made, the different cut-off levels for FeNO and sputum eosinophils, the control strategies and how medications were adjusted.
Sensitivity analyses was done post hoc where the study of Szefler et al20 was excluded from the meta-analysis, as study design was slightly different because traditional asthma measures were part of both groups. While the non-significant difference between groups for the primary outcome was upheld, that for the final ICS that favoured controls became non-significant.
Conclusion
The studies included in this review highlight the difficulties involved in tailoring the dose of ICS based on inflammatory markers (FeNO and sputum eosinophils), instead of primarily on clinical symptoms. Tailoring of asthma treatment based on sputum is effective in decreasing asthma exacerbations in adults. However, tailoring of asthma treatment based on FeNO levels has not been shown to be effective in improving asthma outcomes in children and adults. At present, despite their popularity, there is insufficient evidence to advocate their use in routine clinical practice.
Further randomised controlled trials in both adults and children are required. A priori pragmatic issues of clinical practice such as high versus low doses of ICS and, to a lesser extent, eosinophilic versus non-eosinophilic asthma should be considered with costs analysis for each subgroup. Future randomised controlled trials should preferably be parallel multicentre studies and include outcomes of exacerbations, subjective measures (such as scores for asthma control and QoL) as well as objective measures (FEV1, etc.). It is likely that a clear algorithm based on outcomes rather than a single cut-off is required.26 Analysis of costs and possible adverse events of inhaled and oral corticosteroids would also provide additional important information.
References
Footnotes
See Editorial, p 191
Funding Australian Cochrane Airways Group and Royal Children's Hospital Foundation. AC is supported by an NHMRC Practitioner Fellowship (grant no. 545216).
Competing interests None.
Provenance and peer review Not commissioned; externally peer reviewed.
Linked Articles
- Editorial
- Airwaves