Article Text
Abstract
Background: Pulmonary rehabilitation is effective in improving exercise performance and health status in chronic obstructive pulmonary disease (COPD). However, the role of nutritional support in the enhancement of the benefits of exercise training has not been explored. A double blind, randomised, controlled trial of carbohydrate supplementation was undertaken in patients attending outpatient pulmonary rehabilitation.
Methods: 85 patients with COPD were randomised to receive a 570 kcal carbohydrate rich supplement or a non-nutritive placebo daily for the duration of a 7 week outpatient pulmonary rehabilitation programme. Primary outcome measures were peak and submaximal exercise performance using the shuttle walk tests. Changes in health status, body composition, muscle strength, and dietary macronutrient intake were also measured.
Results: Patients in both the supplement and placebo groups increased shuttle walking performance and health status significantly. There was no statistically significant difference between treatment groups in these outcomes. Patients receiving placebo lost weight whereas supplemented patients gained weight. In well nourished patients (BMI >19 kg/m2) improvement in incremental shuttle performance was significantly greater in the supplemented group (mean difference between groups: 27 (95% CI 1 to 53) m, p<0.05). Increases in incremental shuttle performance correlated with increases in total carbohydrate intake.
Conclusions: When universally prescribed, carbohydrate supplementation does not enhance the rehabilitation of patients with COPD. This study suggests that exercise training results in negative energy balance that can be overcome by supplementation and that, in selected patients, this may improve the outcome of training. The finding of benefit in well nourished patients may suggest a role for nutritional supplementation beyond the treatment of weight loss in COPD.
- nutrition
- exercise performance
- chronic obstructive pulmonary disease
Statistics from Altmetric.com
Chronic obstructive pulmonary disease (COPD) is an important cause of disability and handicap worldwide.1 Patients with COPD are frequently unable to carry out many activities of daily living because of poor exercise performance. Improving physical performance is therefore an important therapeutic goal. Pulmonary rehabilitation is effective in improving exercise performance and health status in COPD.2,3 Exercise training is a core component of pulmonary rehabilitation and improvements in physical performance are an important clinical outcome.4 Any intervention that further enhances the physical effects of training might therefore be of benefit.
The importance of nutrition in improving performance in elite sport is now recognised. Athletes are advised to maximise carbohydrate intake during training and before competition.5 Carbohydrate availability may be of even greater importance in physically deconditioned individuals including those with COPD because they are more reliant on carbohydrate sources as fuel for muscular contraction.6 Moreover, exercise may impose greater energy costs in patients with COPD because of increases in the work of breathing and impaired oxidative metabolism in the peripheral muscles.7
Weight loss is a common clinical problem in COPD. Unfortunately, trials of nutritional supplementation in underweight patients have proved disappointing.8 This might in part be due to the inclusion of patients with progressive weight loss due to an exaggerated systemic inflammatory response who respond particularly poorly to nutritional support.9 In addition, there are difficulties achieving adequate calorie intake in elderly subjects who may offset supplementation with a reduction in normal food intake.10 For appetite to be maintained, supplementation may need to be combined with an anabolic stimulus such as exercise. However, few studies have done this and none have explored the effect of carbohydrate supplementation in enhancing the benefits of training. Importantly, the benefits of carbohydrate provision for training and performance in sport are not restricted to underweight individuals. This may also be true for patients with COPD who are not overtly malnourished.
We have performed a double blind, randomised, controlled trial of nutritional supplementation in COPD patients undergoing pulmonary rehabilitation. Because our aim was to augment exercise training, patients randomised to the treatment arm were supplied with a carbohydrate rich supplement. Our hypothesis was that this intervention would enhance the physical outcome of pulmonary rehabilitation and that these benefits would not be confined to underweight patients. A secondary aim of the study was to measure the changes in health status, body weight, and composition resulting from this therapeutic combination.
METHODS
Patients
Patients referred to the pulmonary rehabilitation programme who met clinical and spirometric criteria for COPD11 were assessed consecutively for inclusion in the study. Patients were stable at recruitment and medical treatment had been optimised in the outpatient clinic before referral to rehabilitation. Patients were excluded if they were unsuitable for the exercise component of the programme due to other conditions such as cardiac, neuropsychiatric, or musculoskeletal disorders. Additional exclusion criteria were a diagnosis of diabetes or glucose intolerance and a body mass index (BMI) of >30 kg/m2. These exclusion criteria were applied because it was felt that nutritional supplementation would be inappropriate in these situations. All patients gave written informed consent for participation in the study. Ethical approval for the study was granted by the Leicestershire research ethics committee.
Study design
All patients participated in the outpatient pulmonary rehabilitation programme at Glenfield Hospital. After baseline measurements had been taken, patients were randomised to receive a carbohydrate rich nutritional supplement three times a day for the duration of the rehabilitation programme or a non-nutritive placebo. Treatment was allocated in blocks of four from a preprepared randomisation list. Treatment was allocated and dispensed independently by a member of staff in the pharmacy department who was not involved with the conduct of the study. Both the investigators and the patients were blinded to the treatment allocation. Unblinding of the study did not occur until the last patient had completed their final assessment.
Outcome measurements
Study assessments were made before randomisation and within 1 week of completion of the rehabilitation programme.
Physical performance
Walking performance was measured using the incremental (ISWT) and endurance (ESWT) shuttle walk tests. The ISWT is a symptom limited, maximal, field exercise test.12 Performance in the ISWT has been shown to be predictive of peak oxygen consumption (Vo2),13 and the test is reproducible after a single practice walk.
Endurance performance was measured using the ESWT.14 This is a constant work rate field exercise test. Walking speed is constant and is set at the equivalent of 85% of the predicted peak Vo2 estimated during the ISWT. The total time walked (excluding the warm-up time) is recorded.
Isometric quadriceps strength (QS) was measured. Patients were seated upright with the knee flexed at 90°. A strap was placed around the lower shin and connected by a chain to a pressure transducer (Sprint, Loughborough, UK). The subject was asked to maximally extend the knee against the strap. Two groups of three attempts were performed with 5–10 minutes rest between them. The highest value of the six attempts was recorded.
Isometric handgrip strength (HGS) was measured using a handgrip dynamometer (Takei Instruments, Japan). After a practice attempt, strength was measured twice in each hand. The mean of the highest values in the right and left hand was recorded. Results are expressed as kg force.
Health status
Disease specific health status was measured using the Self Reported Chronic Respiratory Questionnaire (CRQ-SR). This has been developed from the interviewer led CRQ15 to allow patients to complete the questionnaire without the need for a time consuming interview from a member of staff.16 The questionnaire scores four domains—dyspnoea, fatigue, emotion and mastery. The results are presented as mean scores per question in each dimension. The threshold for a clinically significant change for each dimension has been previously identified as 0.5.17
Body weight and composition
Body weight was measured in light clothing using digital scales (Seca, UK) to the nearest 100 g. Height was measured to the nearest centimetre using a wall mounted stadiometer. Body mass index was calculated as weight/height2 (kg/m2).
Body composition was measured using dual energy x ray absorptiometry (DEXA) (Lunar Expert-XL Bone Densitometer, Lunar Radiation Corporation, Madison, USA). This provides a three compartment model of body composition giving values for bone mass, lean mass, and fat mass. These are derived using software provided by the manufacturer (Software Version 1.91). We have recently established the reproducibility of body composition measurements by DEXA over 7 weeks in stable COPD patients.18
Pulmonary rehabilitation programme
Patients attended the outpatient rehabilitation programme at Glenfield Hospital. They attended twice weekly for a total of 14 sessions, lasting at least 7 weeks. Missed sessions were added to the end of the programme.
The endurance training component of the programme comprised weekly sessions of endurance walking exercises together with a home walking programme. Patients were asked to walk at a speed equivalent to 85% of the predicted peak Vo2 achieved during the ISWT at their initial assessment. Walking times were increased progressively during the course of the programme. At each training session adherence to the training programme was monitored, walking speeds checked, and new targets set for walking times.
Patients also performed a weekly circuit of low impact conditioning exercises designed to increase suppleness and flexibility. The duration of these exercises was increased during the programme but there was no specific progressive weight training.
Each rehabilitation session included education sessions covering a range of topics including disease pathology, treatment, diet, and relaxation.
Nutritional supplementation
Patients allocated to the supplement arm of the study were asked to drink a 125 ml supplement (Respifor, Nutricia, Netherlands) three times per day for the duration of their attendance at rehabilitation. The supplement provided 570 kcal daily in the following macronutrient composition: carbohydrate 60%, fat 20%, protein 20%. This supplement was chosen because its macronutrient profile and low volume was thought particularly suitable for the needs of exercising patients. Patients in the placebo group received an identically packaged and flavoured non-nutritive placebo.
Patients were supplied with cartons each week when attending the rehabilitation sessions. At each visit patients were interviewed by a qualified dietician (RLB). During these interviews self-reported compliance, adverse events, and changes to concomitant therapy were recorded.
Dietary intake
The effect of the intervention on normal dietary intake was assessed using a three-day food diary.19 This was performed before randomisation and again during the second half of the rehabilitation programme (weeks 4–7). Standard food portion sizes20 were used to estimate food weights. Mean daily calorie and macronutrient intake was calculated by entering food records into a computerised version of food composition tables (Microdiet version 9.1, University of Salford, UK).21
Data analysis
Mean increases in ISWT performance from our rehabilitation programme are around 50 m. The clinical significance of further increases in performance resulting from treatment adjunctive to rehabilitation is unknown but we judged that an additional increase of 35 m would be of functional benefit to patients. The sample size was calculated (with 80% power) to ensure we would detect this additional increase in ISWT performance in the supplement group, assuming a 25% dropout rate from rehabilitation. To achieve this we needed to recruit 85 patients, with 56 completing the study.
We hypothesised that undernourished patients might respond differently to our intervention. A subgroup analysis of the main study outcomes was therefore made in patients with a BMI above and below 19 kg/m2. This is an accepted lower limit of normal for BMI.22
Because patients who dropped out were unable to attend for repeat assessments, the analysis was based on treatment received. Between group changes for normally distributed variables were compared with analysis of covariance (ANCOVA) using the corresponding baseline value as a covariate (Mann-Whitney U test for non-parametric or ordinal data). Statistical tests were performed using SPSS Version 10 (Chicago, IL, USA).
RESULTS
Eighty five patients were recruited to the study. The treatment groups were well matched at baseline (table 1). The condition and medication of all patients was stable at the time of recruitment. Six patients were taking long term oral corticosteroids (maximum dose 7.5 mg prednisolone daily).
Mean (SD) baseline characteristics of patients entering the study.
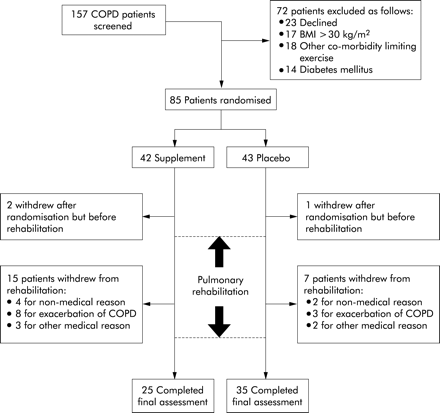
The trial outline is shown in fig 1. Sixty patients completed the trial. More patients dropped out from the supplement group (n=17) than from the placebo group (n=8; p=0.027, χ2 test). Dropouts were due to inability or reluctance to complete rehabilitation rather than refusal to consume supplement or placebo. Reasons for withdrawal are detailed in the trial outline. There was a trend to a greater dropout rate due to exacerbations of COPD in the supplement group (n=8) than in the placebo group (n=3), but this was not statistically significant (p=0.058, χ2 test). One patient withdrew because he was unable to tolerate the cartons of drink but he subsequently also withdrew from rehabilitation. For those completing the trial the median number of rehabilitation sessions missed was one in both groups (supplement group: range 0–5, placebo group: range 0–8). One patient in the supplement group completed the final performance and health status assessments but suffered a ruptured abdominal aortic aneurysm and died before his final DEXA scan.
Trial outline based on completion of shuttle walk tests.
Self-reported compliance with the supplement (% cartons taken/cartons prescribed) was excellent in both groups (supplement group 97.6%, placebo group 98.8%). Overall macronutrient intake increased significantly in the supplement group (table 2), but the effect of supplementation was attenuated by a reduction in food intake. As a result, the mean increase in calorie and macronutrient intake in the supplemented group was equivalent to about 70% of the prescribed supplement.
Within group changes in daily macronutrient intake during study period
Changes after rehabilitation within each treatment group are shown in table 3. There were significant increases in shuttle walking performance in both groups. Although there was a statistically significant increase in quadriceps strength in the supplement group, this was probably too small to be of clinical relevance. Patients in the supplement group gained weight while those in the placebo group lost weight. This was principally due to changes in fat mass. Health status measured by the CRQ-SR increased significantly in all domains in both the supplement group (mean (95% CI) changes; Dyspnoea: 0.7 (0.2 to 1.1), p<0.01; Fatigue: 0.6 (0.3 to 1.0), p<0.05; Emotion: 0.6 (0.3 to 1.0), p<0.01; Mastery: 0.4 (0.01 to 0.8), p<0.05) and the placebo group (Dyspnoea: 1.0 (00.6 to 1.4), p<0.01; Fatigue: 0.7 (0.4 to 1.1), p<0.01; Emotion: 0.5 (0.2 to 0.8), p<0.01; Mastery: 0.9 (0.5 to 1.3), p<0.01).
Within group changes and between group differences in outcome variables after pulmonary rehabilitation
Table 3 also shows the between group differences in changes in outcome variables after rehabilitation. Because there was an uneven dropout rate between the groups, a further analysis of covariance was performed to determine if this explained any differences between treatment groups. When patients who dropped out were compared with those who completed the study, they were found to have generally lower baseline values for age, BMI, FEV1 (% predicted), and quadriceps strength. These variables were therefore used as covariates in this analysis. This did not substantially affect the between group differences or levels of significance in the study as a whole or the subgroup analysis.
Both incremental and endurance shuttle walking performance increased more in the supplement group but these differences were not statistically significant. There were no significant differences in the change in health status between the groups. Weight changes between the supplement and placebo groups were significantly different (table 3). These changes were predominantly in the fat compartment. Changes in ISWT and fat mass correlated with increases in carbohydrate intake (ISWT: r=0.336, p=0.011; fat mass: r=0.337, p=0.010; fig 2).
Relationship between changes in carbohydrate intake and changes in (A) fat mass and (B) ISWT performance in placebo group (open circles) and supplement group (closed circles). CHO=carbohydrate. Pearson correlation used to calculate r values.
Subgroup analysis
The results of a subgroup analysis of patients with BMI >19 kg/m2 are shown in table 4. Fifty two patients fell into this group (22 supplement, 30 placebo). Data on patients with BMI <19 kg/m2 are also presented although the numbers in this group were small (3 supplement, 5 placebo). Baseline characteristics in the well nourished subgroup did not differ between treatment groups. In this subgroup there was a significantly greater increase in ISWT in supplemented patients than in those receiving placebo. The differences in change in ESWT, weight, and fat mass were also of greater magnitude. In addition, the relationship between changes in carbohydrate intake and changes in fat mass (r=0.42, p=0.003) and ISWT (r=0.46, p=0.001) were stronger. Normal food intake was better maintained in the well nourished subgroup resulting in greater increases in carbohydrate intake in the supplemented group than in the placebo group (mean (95% CI) change in carbohydrate: 73 (52 to 93) g v −5 (−21 to 10) g).
Subgroup analysis in patients with BMI above and below 19 kg/m2
DISCUSSION
We report the results of a randomised controlled trial of nutritional support in patients with COPD undergoing pulmonary rehabilitation. This trial differs from previous investigations because improving physical performance rather than nutritional status was the principal aim of the study. The combination of nutritional supplementation and exercise training was successful in increasing weight and energy intake in our patients. In unselected patients nutritional supplementation did not significantly enhance the benefits of pulmonary rehabilitation. However, in well nourished patients (BMI >19 kg/m2) the increase in ISWT after training was significantly greater in the supplemented group. Moreover, the magnitude of changes in ESWT and weight were also greater in well nourished patients. This suggests a potential role for nutritional support in enhancing physical performance in this group of patients.
Our results demonstrate marked differences in the pattern of weight change in the supplement and placebo groups. Rehabilitation resulted in weight loss in the placebo group, whereas those in the supplement group gained weight. Notably, these changes in weight were due to alterations in fat rather than lean mass. Lean mass appeared to increase significantly in the placebo group while in the supplement group it was unchanged. The explanation for this is unclear, but the finding of an isolated within group change needs to be interpreted with caution. Importantly, in contrast to the changes in weight and fat mass, there were no significant between group changes in lean mass.
While loss of lean or fat free mass may be considered to be more relevant to function in COPD, lean mass depletion may not simply be the result of energy imbalance but reflects disordered protein metabolism due to other factors such as muscle disuse, hypoxia, or drug treatment. Many of these factors will not have been addressed by rehabilitation and supplementation. While muscle mass can be readily increased by progressive strength training, this would not be expected from an endurance training programme such as ours.
Although normal dietary intake fell in the supplement group, supplementation successfully increased calorie and macronutrient intake by around 70% of the prescribed supplement. This is in line with previous outpatient supplementation programmes in COPD.23 Changes in fat mass were related to changes in carbohydrate intake across both groups, suggesting that maintenance of normal dietary intake during supplementation was an important factor in its efficacy.
These findings suggest that exercise training results in negative energy balance in many patients, which was overcome by supplementation. Physical activity imposes a high energy cost for patients with COPD.24 The increase in physical activity for many patients who attend rehabilitation is substantial, and dietary calorie intake may be insufficient to meet this new metabolic demand. We speculate that this might also impose a limit on the amount of exercise patients can do. Nutritional supplementation might therefore confer a performance advantage by allowing greater adherence to an exercise training programme. This is supported by our finding that increases in walking performance were related to increases in carbohydrate ingestion.
We believe the improvements in performance in the supplemented group could be meaningful for many patients. The increase in ISWT in well nourished patients was 70% greater in the supplemented group than in those receiving rehabilitation alone. Furthermore, in this subgroup the increase in ESWT was 2 minutes greater in the supplement group, although this difference was not statistically significant. The ESWT is considerably more responsive to endurance training but shows greater biological variability than ISWT performance. Our study was powered to detect changes in ISWT and may therefore have been too small to detect clinically important changes in ESWT.
An improvement in performance from carbohydrate supplementation is biologically plausible. Carbohydrate is an important source of energy for endurance exercise but intramuscular stores are limited. Carbohydrate feeding can prolong endurance in healthy subjects25 and there is evidence that a high carbohydrate diet can enhance the effects of endurance training.26 Muscle glycogen stores may be lower in COPD patients27 and, like other deconditioned individuals, they are likely to be highly reliant on carbohydrate as a source of fuel for muscular contraction.6 If physical activity increases, carbohydrate availability may become an important factor in sustaining exercise.
To our knowledge this is the first study to investigate the performance benefits of carbohydrate supplementation when combined with exercise training in COPD. A recent meta-analysis of clinical trials of nutritional supplementation failed to identify significant improvements in weight or exercise capacity.8 To date, only one trial combining nutritional support and rehabilitation has been reported.28 In this study similar changes in weight and fat mass were seen but there was no performance advantage above that of rehabilitation alone in either depleted or non-depleted patients. One major difference between this study and ours was the composition of the supplement, which was fat rather than carbohydrate rich. It is possible that fat supplementation provides sufficient energy to allow weight gain but does not meet the metabolic demands of exercising muscles.
Our finding of greater benefits from supplementation in well nourished patients contrasts with the traditional aims of nutritional support in COPD. Well nourished patients also showed larger increases in weight and fat mass and better maintenance of dietary calorie intake, suggesting that in these patients the suppression of appetite by supplementation was not as great. Some underweight COPD patients show signs of an exaggerated systemic inflammatory response leading to an increase in resting energy expenditure, appetite suppression, and progressive cachexia.29 These patients appear to respond poorly to nutritional support9 and show appetite suppression in the face of increased energy requirements.30 We conducted a subgroup analysis in patients with a BMI of >19 kg/m2 in an effort to identify and exclude patients that might fall into this “non-responder” category. We hypothesised that these patients might attenuate the effect of the supplementation in the study population as a whole. While the outcome of a subgroup analysis needs to be interpreted with caution, the results do suggest that nutritional support combined with exercise may be beneficial for patients of normal nutritional status. Prospective studies in this group of patients are needed before such treatment can be recommended in routine clinical practice. Conclusions about the effectiveness of supplementation during rehabilitation in underweight patients cannot be drawn from this study because of the small numbers of patients in this subgroup.
The dropout rate for the study is in line with our experience of pulmonary rehabilitation but the rate was greater in the supplemented group. There was also a higher dropout rate due to disease exacerbations, although this difference was just outside statistical significance. This may have occurred by chance, but we cannot rule out the possibility that carbohydrate had an adverse effect on patients suffering an exacerbation because of the increase in carbon dioxide production that results from its oxidation. This effect has been demonstrated experimentally but its clinical relevance is unclear.31 The small volume supplement we used was designed to reduce any impact this might have and be easy for patients to tolerate. Studies using this supplement have indicated there are no short term adverse effects on ventilation or respiratory quotient in COPD patients.32
Pulmonary rehabilitation is established as effective treatment for enhancing performance in COPD. The long term aim of rehabilitation is the maintenance of physical fitness through a more active lifestyle. The implication of our study is that, for some patients, this lifestyle change could also result in negative energy balance and progressive weight loss. Further studies are warranted to examine the long term effects of physical activity on nutritional status and the role of more prolonged nutritional support in this context.
In conclusion, this trial indicates that, when universally prescribed, carbohydrate rich nutritional support does not enhance the rehabilitation of patients with COPD. Our data suggest that exercise training results in negative energy balance that can be overcome by supplementation and that, in selected patients, this may improve the outcome of training. The finding of benefit in well nourished patients may suggest a role for nutritional supplementation beyond the treatment of weight loss in COPD.
Acknowledgments
This study was planned, conducted and analysed independently by the authors. Partial financial support, covering consumable costs and dietetic support, was provided by Nutricia, Zoetermeer, The Netherlands. All authors were independently employed by University Hospitals of Leicester NHS Trust. We are grateful to Dr N Taub at the Trent Institute for Health Services Research for statistical advice.
REFERENCES
Footnotes
-
M Steiner recruited patients to the study, carried out outcome measurements, analysed the data, and wrote the manuscript with the help of the other authors. S Singh was responsible for the running of the pulmonary rehabilitation programme. R Barton provided dietetic support and monitoring for patients who were participating in the study and helped to analyse the data. M Morgan was involved in planning the study and provided overall supervision of the project.
Linked Articles
- Airwaves