Revised: March 8, 2010
Accepted: March 15, 2010
Published online: March 26, 2010
The cardiovascular disease continuum (CVDC) is a sequence of events, which begins from a host of cardiovascular risk factors that consists of diabetes mellitus, dyslipidemia, hypertension, smoking and visceral obesity. If it is not intervened with early, it inexorably progresses to atherosclerosis, coronary artery disease, myocardial infarction, left ventricular hypertrophy, and left ventricular dilatation, which lead to left ventricular diastolic or systolic dysfunction and eventually end-stage heart failure and death. Treatment intervention at any stage during its course will either arrest or delay its progress. In this editorial, the cardiovascular risk factors that initiate and perpetuate the CVDC are briefly discussed, with an emphasis on their early prevention or aggressive treatment.
- Citation: Chrysant SG. Stopping the cardiovascular disease continuum: Focus on prevention. World J Cardiol 2010; 2(3): 43-49
- URL: https://www.wjgnet.com/1949-8462/full/v2/i3/43.htm
- DOI: https://dx.doi.org/10.4330/wjc.v2.i3.43
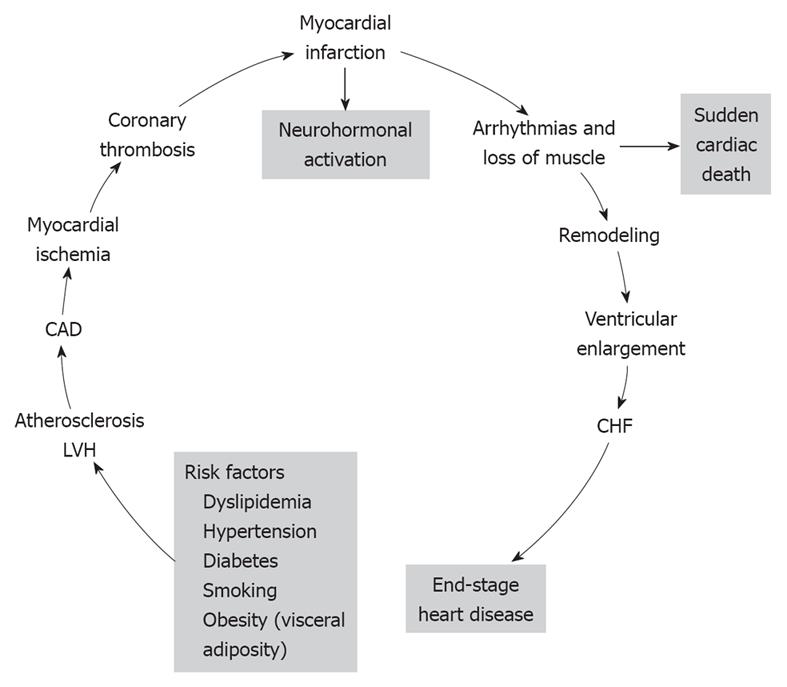
The cardiovascular disease continuum (CVDC) was first conceived by Dzau et al[1] in 1991; it is a chain of events precipitated by several cardiovascular risk factors, which if left untreated, inexorably culminate in end-stage heart failure (HF) and death. The major cardiovascular risk factors that lead to the CVDC are listed at the bottom of Figure 1 and consist of dyslipidemia, hypertension, diabetes, obesity and smoking[2]. All these risk factors, with the exception of smoking, constitute the metabolic syndrome. The metabolic syndrome is defined by the coexistence of any three of the following risk factors: (1) increased waist circumference (≥ 102 cm in men, ≥ 88 cm in women); (2) high triglyceride levels (≥ 150 mg/dL, ≥ 1.68 mmol/L); (3) high-density lipoprotein cholesterol (HDL-C; < 40 mg/dL, < 1.03 mm/L in men, or < 50 mg/dL, < 1.29 mmol/L in women); (4) blood pressure (BP; ≥ 130/85 mmHg); and (5) glucose (≥ 100 mg/dL, ≥ 5.5 mmol/L); and is associated with high incidence of CVD[3]. Since its introduction, the CVDC has been validated by several clinical trials and epidemiological studies, which have provided new insights into its underlying pathophysiology and the possible arrest of its progression by early intervention[4]. Mounting evidence suggests that early intervention in managing the cardiovascular risk factors is more important than treating the CVD itself[4]. CVD complications take years to develop, therefore, this affords ample time for early intervention and treatment of the various cardiovascular risk factors. In this editorial, the main CVD risk factors and their treatment are briefly reviewed.
High cholesterol levels have long been considered an independent risk factor for CVD, and total cholesterol levels of 200 mg/dL (5.17 mmol/L) or higher and low-density lipoprotein cholesterol (LDL-C) levels of 130 mg/dL (3.36 mmol/L) or higher have been found in 50.7% and 45.8% of adult subjects, respectively[5]. In addition, with the increase in obesity, total cholesterol levels > 200 mg/dL (5.17 mmol/L) have been found in 10% of children aged 12-19 years old, and of those screened, only 28.6% knew that high cholesterol is a risk factor for CVD. Also, a recent analysis of data for the United States, Finland and Australia[6] has found that adolescents with dyslipidemia ≥ 95th percentile have a higher incidence of increased carotid intima-media thickness in adulthood, which is a progenitor of coronary artery disease (CAD) in later life[7]. These data suggest that obese adolescents with or without hypertension should be routinely screened for dyslipidemia and treated with lifestyle modification, or more aggressively, with cholesterol-lowering drugs. Adults with dyslipidemia and preexisting CAD should also be aggressively treated according to ATP III guidelines, to LDL-C < 130 mg/dL (3.36 mmol/L) for moderate CVD risk, to < 100 mg/dL (2.59 mmol/L) for high risk, and to < 70 mg/dL (1.81 mmol/L) for very high CVD risk[8]. Several recent outcome trials have shown that aggressive treatment of LDL-C to < 70 mg/dL (1.81 mmol/L) with statins provides protection against recurrent CAD in high risk patients[9-11]. However, despite aggressive LDL-C lowering, CVD continues to increase. According to the American Heart Association statistics, the incidence of CVD increased by 12% from 70.1 million in 2005 to 79.4 million in 2007[12]. Therefore, besides LDL-C, other lipid subclasses have been considered as culprits for this increase in CVD, and recently, high non-HDL-C levels have been the focus for this increase, and have suggested that dyslipidemia is a multifactorial disease and should be treated with a combination of statins and other drugs[13]. Also, the atherogenic phenotype that consists of small dense LDL-C particles, low HDL-C and increased triglyceride levels is also associated with high incidence of coronary heart disease[14].
Diabetes mellitus, especially type 2, accounts for > 97% of the adult diabetic population and its prevalence has increased from 5% in 1988 to 6.5% in 2002, and is in line with the 6% estimate of global prevalence[15]. This rise has been attributed to the increasing incidence of obesity and the aging of the population, which accounts for an annual incidence of 1.3 million Americans with new-onset type 2 diabetes and for the 18.2 million Americans with type 2 diabetes in 2002[5]. The incidence of type 2 diabetes is also rising rapidly in Asian children and adults[16]. Recent reports from China, Japan and the Pacific Islands indicate that > 70% of children are diagnosed with type 2 diabetes. In addition, a recent study from 10 Asian countries has suggested that Asian children are more susceptible in developing diabetes than Caucasian[17]. Overt diabetes mellitus evolves from a pre-diabetic state that is characterized by insulin resistance, impaired glucose tolerance and fasting plasma glucose levels of 100-125 mg/dL (5.55-6.94 mmol/L). Several clinical trials have reported a significant improvement in the metabolic status, a delay in the progression of pre-diabetes to overt diabetes, and a decrease in the incidence of cardiovascular events with a combination of diet, exercise and anti-diabetic drugs, if necessary[18-20]. Overt type 2 diabetes mellitus is a serious CVD risk factor and is presently considered a “cardiovascular risk equivalent”, thus conferring to diabetic patients the same risk for future cardiovascular complications as those who have already sustained a prior myocardial infarction (MI)[21]. It is therefore critical that type 2 diabetes mellitus is treated aggressively with a combination of diet, exercise and anti-diabetic drugs, to a level of hemoglobin A1c ≤ 7% and BP < 130/80 mmHg, in order to prevent cardiovascular and renal complications[22,23]. Three recently published studies have shown mixed results with respect to aggressive control of diabetes (hemoglobin A1c < 7%). One study has shown a significant decrease in renal complications and no effect on cardiovascular and stroke complications by reducing hemoglobin A1c to < 7%[24]. Another has shown an increased incidence in total mortality and no effect on cardiovascular events in the intensively treated compared to the standard treated group[25]. A third study has shown no significant difference between the aggressively treated (hemoglobin A1c < 7%) and the standard treated (hemoglobin A1c > 7%) groups[26]. In that study, LDL-C was decreased to 80 mg/dL (2.1 mmol/L). For the time being, it is prudent not to lower hemoglobin A1c to < 7% in high-risk patients until new information becomes available. Also, it is recommended that LDL-C is lowered to < 100 mg/dL (< 2.59 mmol/L) in diabetic patients because they are considered to be high-risk patients[22].
Body overweight and obesity start from an early age. A large epidemiological study of 34 countries involving young persons aged 10-16 years old has shown that overweight and obesity are directly related to television viewing time, lack of exercise, and increased consumption of sweets and soft drinks, and decreased consumption of fruits and vegetables[27]. Countries with the highest obesity rates are the United States, Canada, England and Southwest Europe[27]. Other epidemiological studies have also shown that the percentage of Americans who are either overweight or obese has increased significantly over the past 25 years, and accounts for 64% and 30.5% of subjects age ≥ 20 years who are either overweight or obese, respectively[28]. A rapid rise in obesity and the metabolic syndrome has also been noted in many Asian countries as a result of changes in nutrition and physical activity[29]. This rise has resulted in increased incidence of diabetes and CVD[29]. Excess body weight, besides being an independent risk factor for CVD, also contributes to other risk factors, such as type 2 diabetes mellitus, hypertension and dyslipidemia, which further increase the prevalence and severity of CVD[28,30]. Central obesity is associated with insulin resistance and has been shown to be an independent risk factor for ischemic stroke, even after adjustments for body mass index and other risk factors[31]. Because of these alarming trends in obesity increase, major medical societies have issued recent guidelines instructing healthcare professionals about how to stem the rise in this epidemic, by advising their patients about weight loss, diet, exercise, and pharmacological treatment, if necessary[27,28,32,33].
Cigarette smoking is a well-established risk factor for the CVDC[34,35], and is listed in the Framingham CVD risk factors[36]. Long-term prospective studies have clearly demonstrated the considerable mortality risk reduction associated with smoking cessation[35,37,38]. A 50-year follow-up of 34 439 male British physicians has shown that quitting cigarette smoking at any age is associated with prolongation of life expectancy[35]. In that study, physicians who stopped cigarette smoking at age 60, 50, 40 or 30 years, gained about 3, 6, 9, or 10 years in life expectancy, respectively[35]. A recent study has also shown that subjects with CVD improve their survival[39]. In 1521 patients aged ≤ 65 years with a first MI, who were followed for a mean 13.2 years, the odds of dying were 0.57 for never smokers, and 0.50 for pre-MI quitters compared to persistent smokers. In addition, among the persistent smokers, in those who reduced the number of cigarettes smoked, there was an 18% decline in mortality for every five-cigarette decrease in smoking[39]. The mechanisms by which cigarette smoking exerts its cardiovascular damaging effects is not clearly delineated. The most plausible mechanisms include, lipid oxidation, inflammation and thrombosis, with lipid oxidation being the most dominant[40]. Additional factors include vasospasm from nicotine and decreased oxygen delivery due to formation of carboxyhemoglobin. Quitting cigarette smoking is very difficult and recidivism is very high. According to a current American report, approximately 44% of smokers attempt to quit annually, but only 4%-7% succeed[41]. The smoking cessation rate is a little higher in persons who suffer from CAD. It has been estimated that between 28% and 74% quit after an acute MI[42,43]. However, about 40% of the quitters relapse and the major reason is post-MI depression[42]. Medical intervention has resulted in a higher percentage of patients who quit smoking: 61% vs 42% of controls[43]. Smoking cessation or abstinence altogether has been a major undertaking by many countries, by forbidding cigarette smoking in closed places or increasing the price of cigarettes. Family guidance and student education in schools about the health hazards of cigarette smoking will help stop young people from taking up cigarette smoking.
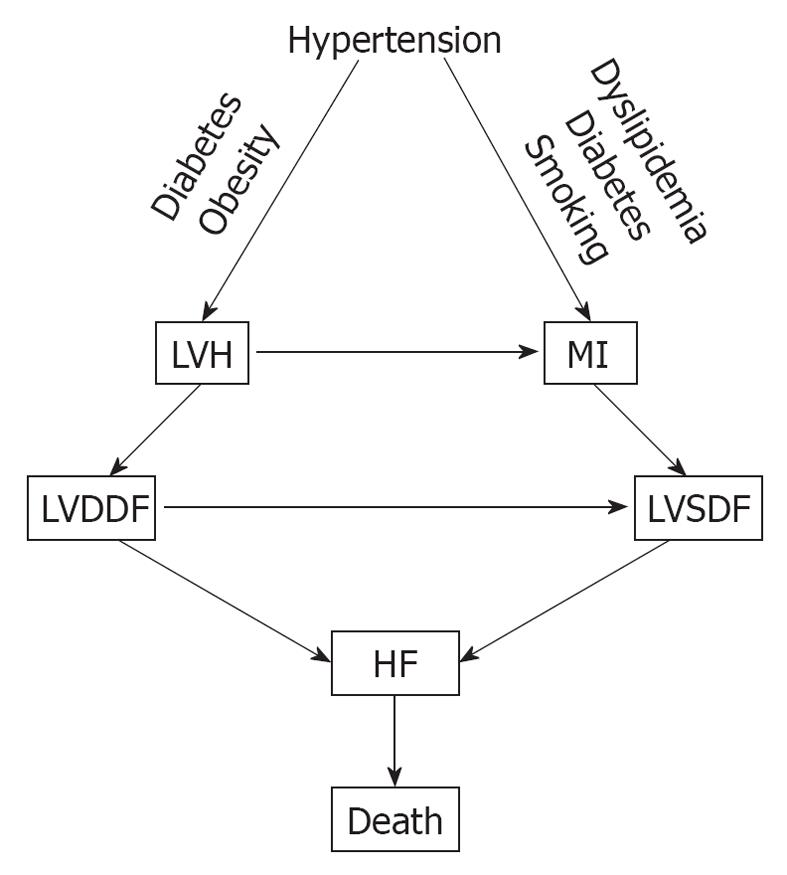
Hypertension is one of the major cardiovascular risk factors for the CVDC and its incidence continues to rise. It increased by 10% between 2005 and 2007, from 65 million to 72 million[12]. Hypertension evolves from a pre-hypertensive state, and this evolution can be delayed or prevented by treating pre-hypertension with diet, salt restriction or drugs[44-48]. There is a linear and continuous relationship between BP level and cardiovascular morbidity and mortality, regardless of age or sex[49], and its reduction is also directly related to the decreased incidence of cardiovascular and cerebrovascular complications[50,51]. In addition, hypertension is one of the most common conditions that predispose to HF. A recent meta-analysis of clinical trials of 193 424 patients has found that 24 837 patients suffered major cardiovascular events[52]. Of these, 7171 (28.9%) were cases of HF, 10 223 (41.1%) were cases of CAD, and 7443 (30.0%) were stroke cases. The incidence of HF was similar to that of stroke and was more prevalent in older persons (> 65 years), in blacks, and in diabetics. Other investigators have also reported that hypertension is a major risk factor for HF[53,54]. In a meta-analysis by Moser et al[53], of 13 342 subjects with hypertension, 1493 (11.2%) from the control groups progressed from less severe to severe hypertension, compared with only 95 of 13 389 (0.17%) from the treated groups. The incidence of left ventricular hypertrophy (LVH) and HF was higher in the control (placebo) than in the treated groups[53]. In the Framingham Heart Study, the association of baseline systolic, diastolic and pulse pressures was examined in 2040 subjects aged 50-79 years old who were free from HF at the baseline examination, and HF developed in 11.8% after 24 years of observation[54]. All these studies point to a common pathophysiological mechanism for the development of HF. Untreated or poorly treated hypertension, alone or in combination with obesity and diabetes mellitus, eventually leads to cardiac remodeling, LVH, left ventricular diastolic dysfunction (LVDDF), HF and death. In addition, LVDDF may also lead to left ventricular systolic dysfunction, which also can arise from MI as a result of hypertension, dyslipidemia and diabetes. This eventually leads to HF and death as depicted in Figure 2. It is, therefore, prudent that instead of focusing on treating the end-stage disease, our attention should be directed to the early diagnosis and treatment of hypertension and other comorbid conditions. There are several options for the treatment of hypertension, including diet[48], salt restriction[47], or drug therapy with any class of antihypertensive drugs, but preferably with drugs that block the renin angiotensin system (RAS), such as angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs) and direct renin inhibitors (DRIs). These drugs are more effective in preventing or regressing cardiac remodeling and LVH[55-60].
From the evidence presented, it appears that early detection and treatment of the risk factors that initiate the CVDC could stop or greatly delay its further progression. The emphasis, therefore, should be based on preventing the disease, instead of waiting for it to develop and then treat it. The new treatment paradigm is a shift to the left on the events that comprise the CVDC (Figure 1). On this theme, there have been several recent calls to practicing physicians by national scientific committees for proactive treatment of cardiovascular risk factors[27,30,32]. There is an urgent need to stem the rising tide of obesity and the metabolic syndrome and their consequences by stressing weight loss through diet and exercise, starting from childhood and continuing through adult life. Pre-diabetes should be recognized and treated early to prevent its progression to overt diabetes. Overt diabetes should be treated aggressively to hemoglobin A1c < 7%, with a combination of diet, exercise and anti-diabetic drugs. Caution should be exercised in older, high-risk patients to avoid serious hypoglycemia. High cholesterol level should also be recognized and treated early, and cigarette smoking should be discouraged through parental guidance and school education about the serious health problems that it can cause later in life. Above all, hypertension should be diagnosed and brought under control early, before it causes target organ damage that is difficult to repair. Whether to treat pre-hypertension pharmacologically, on a large scale, is a question that needs to be addressed soon. Preliminary studies have shown that treatment of pre-hypertension can delay or stop its progression to overt hypertension. Non pharmacological means such as weight loss, salt restriction and exercise should be tried first because they are known to work. Recent reports that aggressive treatment of hypertension is associated with higher cardiovascular complications concerns older, high-risk subjects with preexisting CAD[61,62]. Aggressive control of uncomplicated hypertension is very important because it prevents target organ damage and the incidence of stroke, HF and renal failure. There are several classes of antihypertensive drugs to choose from because they are all effective in lowering BP[63], but individualization of treatment may be necessary. Diuretics, although effective in lowering BP, may not be a good choice for hypertensive subjects with diabetes or the metabolic syndrome, because they increase blood glucose, which is associated with high incidence of cardiovascular morbidity and mortality[64-66]. Drugs that block the RAS such as ACEIs, ARBs, and DRIs are preferable in such cases, because they interfere with the action of angiotensin II, which is responsible for cardiovascular remodeling, new-onset diabetes mellitus, and HF (Figure 2). In the Acute Decompensated Heart Failure National Registry (ADHERE), 91% of patients with HF and preserved ejection fraction had hypertension, CAD and diabetes[67]. In the Antihypertensive and Lipid-Lowering Treatment to prevent Heart Attack Trial (ALLHAT), control of BP was the most important factor in the reduction of hospitalization for HF[68]. Since these comorbidities that are associated with hypertension greatly influence the patient’s outcome, clinicians should try to identify and treat aggressively all these conditions associated with hypertension.
Peer reviewers: Paul Erne, MD, Professor, Head, Department of Cardiology, Luzerner Kantonsspital, CH-6000 Luzern 16, Switzerland; Carmine Gazzaruso, MD, PhD, Section of Cardiovascular & Metabolic Diseases, Center for Applied Clinical Research (Ce.R.C.A.), Clinical Institute, "Beato Matteo" ICBM, Corso Pavia, 84, 27029 Vigevano, Italy; Filippo Cademartiri, MD, PhD, Department of Radiology - c/o Piastra Tecnica - Piano 0, Azienda Ospedaliero-Universitaria di Parma, Via Gramsci, 14 - 43100 Parma, Italy
S- Editor Cheng JX L- Editor Kerr C E- Editor Zheng XM
| 1. | Dzau V, Braunwald E. Resolved and unresolved issues in the prevention and treatment of coronary artery disease: a workshop consensus statement. Am Heart J. 1991;121:1244-1263. [Cited in This Article: ] |
| 2. | Dzau VJ, Antman EM, Black HR, Hayes DL, Manson JE, Plutzky J, Popma JJ, Stevenson W. The cardiovascular disease continuum validated: clinical evidence of improved patient outcomes: part I: Pathophysiology and clinical trial evidence (risk factors through stable coronary artery disease). Circulation. 2006;114:2850-2870. [Cited in This Article: ] |
| 3. | Grundy SM. Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab. 2004;89:2595-2600. [Cited in This Article: ] |
| 4. | Chrysant SG. Angiotensin II receptor blockers in the treatment of the cardiovascular disease continuum. Clin Ther. 2008;30 Pt 2:2181-2190. [Cited in This Article: ] |
| 5. | Eyre H, Kahn R, Robertson RM, Clark NG, Doyle C, Hong Y, Gansler T, Glynn T, Smith RA, Taubert K. Preventing cancer, cardiovascular disease, and diabetes: a common agenda for the American Cancer Society, the American Diabetes Association, and the American Heart Association. Circulation. 2004;109:3244-3255. [Cited in This Article: ] |
| 6. | Magnussen CG, Venn A, Thomson R, Juonala M, Srinivasan SR, Viikari JS, Berenson GS, Dwyer T, Raitakari OT. The association of pediatric low- and high-density lipoprotein cholesterol dyslipidemia classifications and change in dyslipidemia status with carotid intima-media thickness in adulthood evidence from the cardiovascular risk in Young Finns study, the Bogalusa Heart study, and the CDAH (Childhood Determinants of Adult Health) study. J Am Coll Cardiol. 2009;53:860-869. [Cited in This Article: ] |
| 7. | Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115:459-467. [Cited in This Article: ] |
| 8. | Grundy SM, Cleeman JI, Merz CN, Brewer HB Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC Jr, Stone NJ. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227-239. [Cited in This Article: ] |
| 9. | Murphy SA, Cannon CP, Wiviott SD, McCabe CH, Braunwald E. Reduction in recurrent cardiovascular events with intensive lipid-lowering statin therapy compared with moderate lipid-lowering statin therapy after acute coronary syndromes from the PROVE IT-TIMI 22 (Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis In Myocardial Infarction 22) trial. J Am Coll Cardiol. 2009;54:2358-2362. [Cited in This Article: ] |
| 10. | Tikkanen MJ, Szarek M, Fayyad R, Holme I, Cater NB, Faergeman O, Kastelein JJ, Olsson AG, Larsen ML, Lindahl C. Total cardiovascular disease burden: comparing intensive with moderate statin therapy insights from the IDEAL (Incremental Decrease in End Points Through Aggressive Lipid Lowering) trial. J Am Coll Cardiol. 2009;54:2353-2357. [Cited in This Article: ] |
| 11. | LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, Gotto AM, Greten H, Kastelein JJ, Shepherd J. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425-1435. [Cited in This Article: ] |
| 12. | Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69-e171. [Cited in This Article: ] |
| 13. | Arsenault BJ, Rana JS, Stroes ES, Després JP, Shah PK, Kastelein JJ, Wareham NJ, Boekholdt SM, Khaw KT. Beyond low-density lipoprotein cholesterol: respective contributions of non-high-density lipoprotein cholesterol levels, triglycerides, and the total cholesterol/high-density lipoprotein cholesterol ratio to coronary heart disease risk in apparently healthy men and women. J Am Coll Cardiol. 2009;55:35-41. [Cited in This Article: ] |
| 14. | Koba S, Hirano T, Ito Y, Tsunoda F, Yokota Y, Ban Y, Iso Y, Suzuki H, Katagiri T. Significance of small dense low-density lipoprotein-cholesterol concentrations in relation to the severity of coronary heart diseases. Atherosclerosis. 2006;189:206-214. [Cited in This Article: ] |
| 15. | Adeghate E, Schattner P, Dunn E. An update on the etiology and epidemiology of diabetes mellitus. Ann N Y Acad Sci. 2006;1084:1-29. [Cited in This Article: ] |
| 16. | Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon KH, Hu FB. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301:2129-2140. [Cited in This Article: ] |
| 17. | Cockram CS. Diabetes mellitus: perspective from the Asia-Pacific region. Diabetes Res Clin Pract. 2000;50 Suppl 2:S3-S7. [Cited in This Article: ] |
| 18. | Kosaka K, Noda M, Kuzuya T. Prevention of type 2 diabetes by lifestyle intervention: a Japanese trial in IGT males. Diabetes Res Clin Pract. 2005;67:152-162. [Cited in This Article: ] |
| 19. | Eriksson KF, Lindgärde F. No excess 12-year mortality in men with impaired glucose tolerance who participated in the Malmö Preventive Trial with diet and exercise. Diabetologia. 1998;41:1010-1016. [Cited in This Article: ] |
| 20. | Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. 2002;359:2072-2077. [Cited in This Article: ] |
| 21. | Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229-234. [Cited in This Article: ] |
| 22. | Buse JB, Ginsberg HN, Bakris GL, Clark NG, Costa F, Eckel R, Fonseca V, Gerstein HC, Grundy S, Nesto RW. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care. 2007;30:162-172. [Cited in This Article: ] |
| 23. | American Diabetes Association. Standards of medical care in diabetes--2008. Diabetes Care. 2008;31 Suppl 1:S12-S54. [Cited in This Article: ] |
| 24. | Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560-2572. [Cited in This Article: ] |
| 25. | Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH Jr. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545-2559. [Cited in This Article: ] |
| 26. | Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129-139. [Cited in This Article: ] |
| 27. | Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898-918. [Cited in This Article: ] |
| 28. | Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999-2000. JAMA. 2002;288:1723-1727. [Cited in This Article: ] |
| 29. | Misra A, Khurana L. The metabolic syndrome in South Asians: epidemiology, determinants, and prevention. Metab Syndr Relat Disord. 2009;7:497-514. [Cited in This Article: ] |
| 30. | Grundy SM, Pasternak R, Greenland P, Smith S Jr, Fuster V. Assessment of cardiovascular risk by use of multiple-risk-factor assessment equations: a statement for healthcare professionals from the American Heart Association and the American College of Cardiology. Circulation. 1999;100:1481-1492. [Cited in This Article: ] |
| 31. | Suk SH, Sacco RL, Boden-Albala B, Cheun JF, Pittman JG, Elkind MS, Paik MC. Abdominal obesity and risk of ischemic stroke: the Northern Manhattan Stroke Study. Stroke. 2003;34:1586-1592. [Cited in This Article: ] |
| 32. | Kumanyika SK, Obarzanek E, Stettler N, Bell R, Field AE, Fortmann SP, Franklin BA, Gillman MW, Lewis CE, Poston WC 2nd. Population-based prevention of obesity: the need for comprehensive promotion of healthful eating, physical activity, and energy balance: a scientific statement from American Heart Association Council on Epidemiology and Prevention, Interdisciplinary Committee for Prevention (formerly the expert panel on population and prevention science). Circulation. 2008;118:428-464. [Cited in This Article: ] |
| 33. | Nilsson PM, Lurbe E, Laurent S. The early life origins of vascular ageing and cardiovascular risk: the EVA syndrome. J Hypertens. 2008;26:1049-1057. [Cited in This Article: ] |
| 34. | Wilhelmsson C, Vedin JA, Elmfeldt D, Tibblin G, Wilhelmsen L. Smoking and myocardial infarction. Lancet. 1975;1:415-420. [Cited in This Article: ] |
| 35. | Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years' observations on male British doctors. BMJ. 2004;328:1519. [Cited in This Article: ] |
| 36. | Kannel WB, McGee D, Gordon T. A general cardiovascular risk profile: the Framingham Study. Am J Cardiol. 1976;38:46-51. [Cited in This Article: ] |
| 37. | Wilson K, Gibson N, Willan A, Cook D. Effect of smoking cessation on mortality after myocardial infarction: meta-analysis of cohort studies. Arch Intern Med. 2000;160:939-944. [Cited in This Article: ] |
| 38. | Critchley JA, Capewell S. Mortality risk reduction associated with smoking cessation in patients with coronary heart disease: a systematic review. JAMA. 2003;290:86-97. [Cited in This Article: ] |
| 39. | Gerber Y, Rosen LJ, Goldbourt U, Benyamini Y, Drory Y. Smoking status and long-term survival after first acute myocardial infarction a population-based cohort study. J Am Coll Cardiol. 2009;54:2382-2387. [Cited in This Article: ] |
| 40. | Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004;43:1731-1737. [Cited in This Article: ] |
| 41. | 2008 PHS Guideline Update Panel, Liaisons, and Staff. Treating tobacco use and dependence: 2008 update U.S. Public Health Service Clinical Practice Guideline executive summary. Respir Care. 2008;53:1217-1222. [Cited in This Article: ] |
| 42. | Perez GH, Nicolau JC, Romano BW, Laranjeira R. Depression: a predictor of smoking relapse in a 6-month follow-up after hospitalization for acute coronary syndrome. Eur J Cardiovasc Prev Rehabil. 2008;15:89-94. [Cited in This Article: ] |
| 43. | van Berkel TF, Boersma H, Roos-Hesselink JW, Erdman RA, Simoons ML. Impact of smoking cessation and smoking interventions in patients with coronary heart disease. Eur Heart J. 1999;20:1773-1782. [Cited in This Article: ] |
| 44. | Julius S, Nesbitt SD, Egan BM, Weber MA, Michelson EL, Kaciroti N, Black HR, Grimm RH Jr, Messerli FH, Oparil S. Feasibility of treating prehypertension with an angiotensin-receptor blocker. N Engl J Med. 2006;354:1685-1697. [Cited in This Article: ] |
| 45. | Lüders S, Schrader J, Berger J, Unger T, Zidek W, Böhm M, Middeke M, Motz W, Lübcke C, Gansz A. The PHARAO study: prevention of hypertension with the angiotensin-converting enzyme inhibitor ramipril in patients with high-normal blood pressure: a prospective, randomized, controlled prevention trial of the German Hypertension League. J Hypertens. 2008;26:1487-1496. [Cited in This Article: ] |
| 46. | Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206-1252. [Cited in This Article: ] |
| 47. | He FJ, MacGregor GA. A comprehensive review on salt and health and current experience of worldwide salt reduction programmes. J Hum Hypertens. 2009;23:363-384. [Cited in This Article: ] |
| 48. | Liebson PR, Grandits GA, Dianzumba S, Prineas RJ, Grimm RH Jr, Neaton JD, Stamler J. Comparison of five antihypertensive monotherapies and placebo for change in left ventricular mass in patients receiving nutritional-hygienic therapy in the Treatment of Mild Hypertension Study (TOMHS). Circulation. 1995;91:698-706. [Cited in This Article: ] |
| 49. | Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903-1913. [Cited in This Article: ] |
| 50. | Staessen JA, Li Y, Thijs L, Wang JG. Blood pressure reduction and cardiovascular prevention: an update including the 2003-2004 secondary prevention trials. Hypertens Res. 2005;28:385-407. [Cited in This Article: ] |
| 51. | Turnbull F, Neal B, Pfeffer M, Kostis J, Algert C, Woodward M, Chalmers J, Zanchetti A, MacMahon S. Blood pressure-dependent and independent effects of agents that inhibit the renin-angiotensin system. J Hypertens. 2007;25:951-958. [Cited in This Article: ] |
| 52. | Tocci G, Sciarretta S, Volpe M. Development of heart failure in recent hypertension trials. J Hypertens. 2008;26:1477-1486. [Cited in This Article: ] |
| 53. | Moser M, Hebert PR. Prevention of disease progression, left ventricular hypertrophy and congestive heart failure in hypertension treatment trials. J Am Coll Cardiol. 1996;27:1214-1218. [Cited in This Article: ] |
| 54. | Haider AW, Larson MG, Franklin SS, Levy D. Systolic blood pressure, diastolic blood pressure, and pulse pressure as predictors of risk for congestive heart failure in the Framingham Heart Study. Ann Intern Med. 2003;138:10-16. [Cited in This Article: ] |
| 55. | Dahlöf B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe-Pedersen O. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995-1003. [Cited in This Article: ] |
| 56. | Malmqvist K, Ohman KP, Lind L, Nyström F, Kahan T. Long-term effects of irbesartan and atenolol on the renin-angiotensin-aldosterone system in human primary hypertension: the Swedish Irbesartan Left Ventricular Hypertrophy Investigation versus Atenolol (SILVHIA). J Cardiovasc Pharmacol. 2003;42:719-726. [Cited in This Article: ] |
| 57. | Thürmann PA, Kenedi P, Schmidt A, Harder S, Rietbrock N. Influence of the angiotensin II antagonist valsartan on left ventricular hypertrophy in patients with essential hypertension. Circulation. 1998;98:2037-2042. [Cited in This Article: ] |
| 58. | Cuspidi C, Muiesan ML, Valagussa L, Salvetti M, Di Biagio C, Agabiti-Rosei E, Magnani B, Zanchetti A. Comparative effects of candesartan and enalapril on left ventricular hypertrophy in patients with essential hypertension: the candesartan assessment in the treatment of cardiac hypertrophy (CATCH) study. J Hypertens. 2002;20:2293-2300. [Cited in This Article: ] |
| 59. | Solomon SD, Appelbaum E, Manning WJ, Verma A, Berglund T, Lukashevich V, Cherif Papst C, Smith BA, Dahlöf B. Effect of the direct Renin inhibitor aliskiren, the Angiotensin receptor blocker losartan, or both on left ventricular mass in patients with hypertension and left ventricular hypertrophy. Circulation. 2009;119:530-537. [Cited in This Article: ] |
| 60. | Chrysant SG. Vascular remodeling: the role of angiotensin-converting enzyme inhibitors. Am Heart J. 1998;135:S21-S30. [Cited in This Article: ] |
| 61. | Messerli FH, Panjrath GS. The J-curve between blood pressure and coronary artery disease or essential hypertension: exactly how essential? J Am Coll Cardiol. 2009;54:1827-1834. [Cited in This Article: ] |
| 62. | Sleight P, Redon J, Verdecchia P, Mancia G, Gao P, Fagard R, Schumacher H, Weber M, Böhm M, Williams B. Prognostic value of blood pressure in patients with high vascular risk in the Ongoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial study. J Hypertens. 2009;27:1360-1369. [Cited in This Article: ] |
| 63. | Turnbull F, Neal B, Ninomiya T, Algert C, Arima H, Barzi F, Bulpitt C, Chalmers J, Fagard R, Gleason A. Effects of different regimens to lower blood pressure on major cardiovascular events in older and younger adults: meta-analysis of randomised trials. BMJ. 2008;336:1121-1123. [Cited in This Article: ] |
| 64. | Elliott WJ, Meyer PM. Incident diabetes in clinical trials of antihypertensive drugs: a network meta-analysis. Lancet. 2007;369:201-207. [Cited in This Article: ] |
| 65. | Dunder K, Lind L, Zethelius B, Berglund L, Lithell H. Increase in blood glucose concentration during antihypertensive treatment as a predictor of myocardial infarction: population based cohort study. BMJ. 2003;326:681. [Cited in This Article: ] |
| 66. | Cooper-DeHoff RM, Wen S, Beitelshees AL, Zineh I, Gums JG, Turner ST, Gong Y, Hall K, Parekh V, Chapman AB. Impact of abdominal obesity on incidence of adverse metabolic effects associated with antihypertensive medications. Hypertension. 2010;55:61-68. [Cited in This Article: ] |
| 67. | Shah SJ, Gheorghiade M. Heart failure with preserved ejection fraction: treat now by treating comorbidities. JAMA. 2008;300:431-433. [Cited in This Article: ] |
| 68. | ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288:2981-2997. [Cited in This Article: ] |