Abstract
Thiopurine drug metabolism is a quintessential case of pharmacogenetics. A wealth of experimental and clinical data on polymorphisms in the thiopurine metabolizing enzyme thiopurine methyl transferase (TPMT) has been generated in the past decade. Pharmacogenetic testing prior to thiopurine treatment is already being practiced to some extent in the clinical context, and it is likely that it will be among the first pharmacogenetic tests applied on a regular basis.
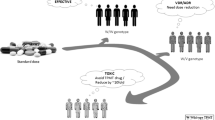
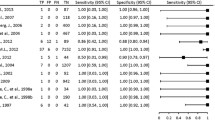
We analyzed the published TPMT data and identified some lessons to be learned for the future implementation of pharmacogenetics for thiopurines as well as in other fields. These include the need for comprehensive and unbiased data on allele frequencies relevant to a broad range of populations worldwide. The nature and frequency of TPMT gene polymorphisms in some ethnic groups is still a matter of speculation, as the vast majority of studies on TPMT allele distribution are limited to only a small subset of alleles and populations. Secondly, an appreciation of the limits of pharmacogenetics is warranted, as pharmacogenetic testing can help in avoiding some, but by far not all adverse effects of drug therapy. An analysis of six clinical studies correlating adverse thiopurine effects and TPMT genotype revealed that an average of 78% of adverse drug reactions were not associated with TPMT polymorphisms. Pharmacogenetic testing will thus not eliminate the need for careful clinical monitoring of adverse drug reactions. Finally, a careful approach toward dose increases for patients with high enzyme activity is necessary, as TPMT-mediated methylation of thiopurines generates a possibly hepatotoxic byproduct.



Similar content being viewed by others
References
Nebert DW, Bingham E. Pharmacogenomics: out of the lab and into the community. Trends Biotechnol 2001; 19: 519–23
Rothstein MA, Epps PG. Ethical and legal implications of pharmacogenomics. Nat Rev Genet 2001; 2: 228–31
Freund CL, Wilfond BS. Emerging ethical issues in pharmacogenomics: from research to clinical practice. Am J PharmacoGenomics 2002; 2(4): 273–81
Weinshilboum R. Thiopurine pharmacogenetics: clinical and molecular studies of thiopurine methyltransferase. Drug Metab Dispos 2001; 29: 601–5
McLeod HL Krynetski EY, Relling MV, et al. Genetic polymorphism of thiopurine methyltransferase and its clinical relevance for childhood acute lymphoblastic leukemia. Leukemia 2000; 14: 567–72
Spire-Vayron de la Moureyre C, Debuysere H, Mastain B, et al. Genotypic and phenotypic analysis of the polymorphic thiopurine S-methyltransferase gene TPMT in a European population. Br J Pharmacol 1998; 125: 879–87
Evans WE, Hon YY, Bomgaars L, et al. Preponderance of thiopurine S-methyl-transferase deficiency and heterozygosity among patients intolerant to mercaptopurine or azathioprine. J Clin Oncol 2001; 19: 2293–301
Krynetski EY, Schuetz JD, Galpin AJ, et al. A single point mutation leading to loss of catalytic activity in human thiopurine S-methytransferase. Proc Natl Acad Sci USA 1995; 92: 949–53
Tai HL, Krynetski EY, Yates CR, et al. Thiopurine S-methyltransferase deficiency: two nucleotide transitions define the most prevalent mutant allele associated with loss of catalytic activity in Caucasians. Am J Hum Genet 1996; 58: 694–702
Otterness D, Szumlanski CL, Wood TC, et al. Human thiopurine methyltransferase pharmacogenetics. J Clin Invest 1998; 101: 1036–44
Hon YY, Fessing MY, Pui CH, et al. Polymorphism of the thiopurine S-methyltransferase gene in African-Americans. Hum Mol Genet 1999; 8: 371–6
Colombel JF, Ferrari N, Debuysere H, et al. Genotypic analysis of thiopurine S-methyltransferase in patients with Crohn’s disease and severe myelosuppression during azathioprine therapy. Gastroenterology 2000; 118: 1025–30
Spire-Vayron de la Moureyre C, Debuysere H, Sabbagh N, et al. Detection of known and new mutations in the thiopurine S-methyltransferase gene by single-strand conformation polymorphism analysis. Hum Mutat 1998; 12: 177–85
Otterness D, Szumlanski C, Lennard L, et al. Human thiopurine methyltransferase pharmacogenetics: gene sequence polymorphisms. Clin Pharmacol Ther 1997; 62: 60–73
Schütz E, von Ahsen N, Oellerich M. Genotyping of eight thiopurine methyltransferase mutations: three-color multiplexing ‘two-color/shared’ anchor and fluorescence-quenching hybridisation probe assays based on thermodynamic nearest-neighbor probe design. Clin Chem 2000; 46: 1728–37
Cuffari C, Theoret Y, Latour S, et al. 6-Mercaptopurine metabolism in Crohn’s disease correlation with efficacy and toxicity. Gut 1996; 39: 401–6
Ameyaw MM, Collie-Duguid ES, Powrie RH, et al. Thiopurine methyltransferase alleles in British and Ghanaian populations. Hum Mol Genet 1999; 8: 367–70
Black AJ, McLeod HL, Capell HA, et al. Thiopurine methyltransferase genotype predicts therapy-limiting severe toxicity from azathioprine. Ann Intern Med 1998; 129: 716–8
McLeod HL, Coulthard S, Thomas A, et al. Analysis of thiopurine methyltransferase variant alleles in childhood acute lymphoblastic leukaemia. Br J Haematol 1999; 105: 696–700
Brouwer C, Marinaki AM, Lambooy LHJ, et al. Pitfalls in determination of mutant alleles of the thiopurine methyltransferase gene. Leukemia 2001; 15: 1792–3
Rossi AM, Bianchi M, Guarnieri C, et al. Genotype-phenotype correlation for thiopurine S-methyltransferase in healthy Italian subjects. Eur J Clin Pharmacol 2001; 57: 51–4
Dervieux T, Medard Y, Verpillat P, et al. Possible implication of thiopurine S-methyltransferase in occurrence of infectious episodes during maintenance therapy of childhood lymphoblastic leukemia with mercaptopurine. Leukemia 2001; 15: 1706–12
Relling MV, Hancock ML, Rivera GK, et al. Mercaptopurine therapy intolerance and heterozygosity at the thiopurine S-methyltransferase gene locus. J Natl Cancer Inst 1999; 91: 2001–8
McLeod HL, Pritchard SC, Githang’a J, et al. Ethnic differences in thiopurine methyltransferase pharmacogenetics evidence for allele specificity in Caucasian and Kenyan individuals. Pharmacogenetics 1999; 9: 773–6
Ando M, Ando Y, Hasegawa Y, et al. Genetic polymorphisms of thiopurine S-methyltransferase and 6-mercaptopurine toxicity in Japanese children with acute lymphoblastic leukemia. Pharmacogenetics 2001; 11: 269–73
Kubota T, Chiba K. Frequencies of thiopurine S-methyltransferase mutant alleles TPMT*2 *3A *3B and *3C in 151 healthy Japanese subjects and the inheritance of TPMT*3C in the family of a propositus. Br J Clin Pharmacol 2001; 51: 475–7
Kumagai K, Hiyama K, Ishioka S, et al. Allelotype frequency of the thiopurine methyltransferase TPMT gene in Japanese. Pharmacogenetics 2001; 11: 275–8
Ishioka S, Hiyama K, Sato H, et al. Thiopurine methyltransferase genotype and the toxicity of azathioprine in Japanese. Intern Med 1999; 38: 944–7
Collie-Duguid ES, Pritchard SC, Powrie RH, et al. The frequency and distribution of thiopurine methyltransferase alleles in Caucasian and Asian populations. Pharmacogenetics 1999; 9: 37–42
Hiratsuka M, Inoue T, Omori F, et al. Detection assay of rare variants of the thiopurine methyltransferase gene by PCR-RFLP using a mismatch primer in a Japanese population. Biol Pharm Bull 2000; 23: 1090–3
Hongeng S, Sasanakul W, Chuansumrit A, et al. Frequency of thiopurine S-methyltransferase genetic variation in Thai children with acute leukemia. Med Pediatr Oncol 2000; 35: 410–4
Schwartz RS. Racial profiling in medical research. N Engl J Med 2001; 344: 1392–3
Lennard L, Van Loon JA, Weinshilboum RM. Pharmacogenetics of acute azathioprine toxicity: relationship to thiopurine methyltransferase genetic polymorphism. Clin Pharmacol Ther 1989; 46: 149–54
Chocair PR, Duley JA, Simmonds HA, et al. The importance of thiopurine methyltransferase activity for the use of azathioprine in transplant recipients. Transplantation 1992; 53: 1051–6
Stolk JN, Boerbooms AMT, de Abreu RA, et al. Reduced thiopurine methyltransferase activity and development of side effects of azathioprine treatment in patients with rheumatoid arthritis. Arthritis Rheum 1998; 41: 1858–66
Lennard L, Lilleyman JS, van Loon J, et al. Genetic variation in response to 6-mercaptopurine for childhood acute lymphoblastic leukaemia. Lancet 1990; 336: 225–9
Naughton MA, Battaglia E, O’Brien S, et al. Identification of thiopurine methyltransferase TPMT polymorphisms cannot predict myelosuppression in systemic lupus erythematosus patients taking azathioprine. Rheumatology 1999; 38: 640–4
Dubinsky MC, Lamothe S, Yang HY, et al. Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology 2000; 118: 705–13
Kirchheiner J, Brøsen K, Dahl ML, et al. CYP2D6 and CYP2C19 genotype-based dose recommendations for antidepressants: a first step towards subpopulation-specific dosages. Acta Psychiatr Scand 2001; 104: 173–92
Acknowledgements
Forschungsschwerpunkt Biotechnik, Gesellschaft und Umwelt, Universitat Hamburg, Hamburg, Germany We thank the German Ministry for Education and Research (BMBF) for research funding and Dr. Paula Bradish for critically reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van Aken, J., Schmedders, M., Feuerstein, G. et al. Prospects and Limits of Pharmacogenetics. Am J Pharmacogenomics 3, 149–155 (2003). https://doi.org/10.2165/00129785-200303030-00001
Published:
Issue Date:
DOI: https://doi.org/10.2165/00129785-200303030-00001