Summary
Abstract
Rituximab (MabThera®, Rituxan®) is an anti-CD20 monoclonal antibody that induces lysis and apoptosis of normal and malignant human B cells, and sensitises malignant B cells to the cytotoxic effect of chemotherapy. In phase III trials in patients with indolent or aggressive B-cell non-Hodgkin’s lymphoma (NHL), intravenous rituximab in combination with chemotherapy was more effective as first- or second-line therapy than chemotherapy alone in providing tumour remission and patient survival. Likewise, in patients with chronic lymphocytic leukaemia (CLL), rituximab in combination with chemotherapy appeared more effective than chemotherapy alone as either first- or second-line treatment. In addition, rituximab maintenance therapy was shown to significantly prolong tumour remission and patient survival in patients with indolent B-cell NHL or CLL. The combination of rituximab with cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) was cost effective as first-line therapy for advanced-stage diffuse large B-cell NHL compared with CHOP alone. Rituximab, either alone or in combination with chemotherapy, was generally well tolerated in patients with NHL or CLL. Overall, rituximab in combination with chemotherapy, is a valuable option for first- and second-line therapy in patients with advanced-stage indolent or aggressive B-cell NHL, and possibly those with B-cell CLL, and is included in current treatment guidelines for these indications. The drug is also potentially useful as maintenance therapy in patients with indolent B-cell NHL or CLL.
Pharmacological Properties
Rituximab is a chimaeric murine/human monoclonal antibody directed against the surface antigen CD20 expressed on all normal and >90% of NHL and =14% of CLL malignant B cells. It induces lysis and apoptosis of all CD20-positive B cells and also sensitises malignant B cells to the cytotoxic effect of chemotherapy. At the recommended dose, rituximab causes rapid and profound B-cell lymphopenia in the majority of patients that lasts for up to 6 months and recovers (from haematopoietic stem cells) within 9–12 months after treatment. As maintenance therapy, rituximab would continue suppression of the B-cell population, including the malignant variety.
Following intravenous administration of rituximab in patients with B-cell NHL, drug serum concentrations increase in a dose-proportional manner (within clinically relevant dosage range), are positively correlated with clinical tumour responses (i.e. they are significantly higher in patients responding to rituximab monotherapy than in nonresponders) and are inversely correlated to the absolute level of circulating peripheral B cells and the tumour bulk measurements at baseline. The pharmacokinetics of rituximab are also characterised by accumulation of the drug with repeated administrations (reflecting the change in the population of CD20-positive B cells) and wide interindividual variability (caused by variable tumour responsiveness and burden between patients). Clinically relevant drug concentrations are present in the serum ≤6 months after, and were also found in the CNS during treatment with rituximab. The pharmacokinetics of rituximab appear to be similar in patients with follicular or diffuse large B-cell NHL, and are unaffected by the coadministration of CHOP.
Significantly lower serum concentrations of rituximab were found in patients with a lymphomatous form of B-cell CLL, compared with values in patients with B-cell NHL.
Therapeutic Efficacy
Indolent NHL: In phase III trials in patients with previously untreated advanced-stage follicular NHL, the addition of rituximab 375 mg/m2 (as an intravenous infusion) to 6–8 cycles of standard chemotherapy regimens was more effective in achieving tumour remission (i.e. complete response [CR]) and short-term (2.5-year) event-free survival (EFS) rates than chemotherapy alone. In combination with cyclophosphamide, vincristine and prednisone (CVP), rituximab also significantly prolonged the duration of tumour response (>2-fold), failure-free (≈4-fold) and disease-free survival (DFS) [>2-fold], and more than doubled the time to disease progression. At the estimated 3-year follow-up, these effects have not yet translated into a significantly greater overall survival (OS) rate compared with CVP alone (89% vs 81%), although both results are clinically relevant. Importantly, however, patients receiving rituximab in addition to CVP spent significantly longer time without disease symptoms or toxicity, and had a substantially improved quality-adjusted survival than patients receiving CVP alone. In patients with advanced follicular NHL, first-line therapy with rituximab in combination with mitoxantrone, chlorambucil and prednisone (MCP) also resulted in significantly longer progression-free survival (PFS) and OS rates than MCP alone.
As a second-line therapy in patients with relapsed or refractory follicular NHL, the addition of a single dose of rituximab to each of six cycles of CHOP or four cycles of fludarabine, cyclophosphamide and mitoxantrone (FCM) regimens significantly improved CR and objective response (OR) rates and prolonged PFS compared with chemotherapy alone in two phase III trials. A significantly improved 4-year OS rate was also reported in a trial with FCM.
The significant survival benefit of adding rituximab to first- or second-line chemotherapy regimens in patients with follicular NHL was confirmed in a meta-analysis of data from several randomised, phase III trials
As monotherapy, four once-weekly intravenous infusions of rituximab 375 mg/m2 produced clinically relevant CR and OR rates in two phase II trials in patients with mucosa-associated lymphoid tissue B-cell NHL. One of the trials showed ≈2-fold longer (p ≈ 0.001) failure-free survival in chemotherapy-naive than in chemotherapy-experienced patients receiving rituximab monotherapy.
Likewise, maintenance therapy with single-agent rituximab 375 mg/m2 (four once-weekly doses repeated every 6 months or single infusion every 2–3 months, for up to 2 years or until relapse), significantly increased CR and/or OR rates, and prolonged duration of remission, EFS and/or PFS after induction therapy in patients with follicular NHL or CLL (lymphomatous form), compared with rituximab re-treatment (at disease progression) [in a phase II trial] or observation (i.e. no further treatment) [in three phase III trials]. Rituximab maintenance therapy provided significantly greater 3- and 4-year PFS and OS rates in both previously treated and untreated patients with follicular NHL, compared with no further treatment. The estimated 3-year OS rates were high with both the rituximab maintenance and re-treatment approach, but results were not statistically significantly different (72% vs 68%) in previously untreated patients.
Aggressive NHL: The combination of rituximab 375 mg/m2 intravenous infusions with a CHOP or a CHOP-like regimen, administered in 6–8 cycles, was more effective than chemotherapy alone as the first-line treatment in patients with advanced-stage, diffuse large B-cell NHL or mantle cell lymphoma (MCL), in several phase III trials. Irrespective of the age of patients with diffuse, large B-cell NHL, the addition of rituximab resulted in significantly greater CR, 2- to 3-year failure-free survival and 2- to 5-year OS rates (the latter effect was observed only in low-risk patients). A significantly greater 5-year PFS rate was also reported with rituximab plus CHOP in a trial in older patients (age ≥60 years) irrespective of disease prognosis. In younger patients (age <60 years) with low-risk disease, rituximab plus CHOP or CHOP-like regimen significantly decreased the relative risk of treatment failure (by 64%) compared with chemotherapy alone.
Significantly greater 1-year failure-free or 4-year OS rates were observed in patients with MCL receiving intravenous infusions of rituximab 375 mg/m2 in combination with first-line CHOP (six cycles) or second-line FCM (four cycles), than those receiving chemotherapy alone, in two randomised, phase III trials. Meta-analysis of data from both trials confirmed the OS benefit of adding rituximab to chemotherapy in patients with MCL, both previously untreated or those with relapsed or refractory MCL.
Rituximab did not improve either tumour response or survival rates in patients with HIV-related, aggressive B-cell NHL, while increasing the risk of infectious death, in a randomised, phase III trial. By contrast, the findings of previous phase I and II trials suggested that rituximab therapy may be beneficial in this patient population.
Rituximab maintenance therapy was effective in patients with diffuse large B-cell NHL, only when the drug was not part of an induction regimen, and was ineffective in patients with MCL.
CLL: Standard rituximab monotherapy (i.e. four intravenous infusions of 375 mg/m2 once weekly) was less effective than fludarabine monotherapy in phase II trials in patients with B-cell CLL. However, the addition of rituximab 375 or 500 mg/m2 to six cycles of fludarabine (with or without cyclophosphamide) significantly improved response (CR and/or OR) and survival (2- to 4-year PFS and/or OS) rates in these patients, compared with chemotherapy alone in retrospective comparative analyses of several phase II and III trials.
Tolerability
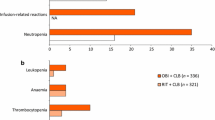
Rituximab, alone or in combination with various chemotherapy regimens, was generally well tolerated in clinical trials in patients with advanced-stage indolent or aggressive B-cell NHL or B-cell CLL. The most common types of adverse events in these trials were infusion-related reactions, haematological adverse events and infections. Infusion-related reactions that occur in the majority of patients, most within 2 hours of the first infusion, are generally mild to moderate flu-like symptoms that usually resolve upon slowing or stopping the infusion, and become less frequent with subsequent infusions. Severe (grade 3/4) reactions, including severe cytokine release syndrome, occur in ≈10% of patients and may also require supportive care (e.g. analgesic, antihistamine, oxygen, intravenous fluids, bronchodilators, vasopressors and/or corticosteroids).
In comparison with chemotherapy, rituximab is associated with a substantially lower incidence of severe neutropenia and infections. Furthermore, the addition of rituximab does not increase the toxicity of chemotherapy in patients with NHL or CLL of B-cell origin. The tolerability profile of rituximab, in combination with chemotherapy, is similar in patients with indolent or aggressive NHL. In addition, the US manufacturer’s prescribing information for rituximab includes boxed warnings for fatal infusion reactions, tumour lysis syndrome and severe mucocutaneous reactions.












Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Jemal A, Murray T, Ward E, et al. Cancer statistics, 2005. CA Cancer J Clin Jan/Feb 2005; 55(1): 10–30
Evans LS, Hancock BW. Non-Hodgkin lymphoma. Lancet. 362 2003 Jul 12; 362: 139–46
Kasper DL, Braunwald E, Fauci AS, et al. Harrison’s principles of internal medicine, 16th Ed.; Chapter 97: Malignancies of lymphoid cells [online]. Available from URL: http://www.harisonsonline.com [Accessed 2005 Aug 29]
National Cancer Institute. Adult non-Hodgkin’s lymphoma (PDQ®): treatment (health professional version) [online]. Available from URL: http://www.cancer.gov/cancerinfo/pdq/treatment/adult-non-hodgkins/healthprofessional [Accessed 2005 Sep 5]
Zinzani PL. Lymphoma: diagnosis, staging, natural history, and treatment strategies. Semin Oncol 2005 Feb; 32(1 Pt 2): 4–10
Miller TP, Leblanc M, Spier C, et al. CHOP alone compared to CHOP plus radiotherapy for early stage aggressive non-Hodgkin’s lymphomas: update of the Southwest Oncology Group (SWOG) randomized trial [abstract no. 3024]. Blood 2001 Nov 16; 98 (11 Part 1 of 2): 724–5a
Oscier D, Fegan C, Hillmen P, et al. Guidelines on the diagnosis and management of chronic lymphocytic leukaemia. Br J Haematol 2004; 125(3): 294–317
Plosker GL, Figgitt DP. Rituximab: a review of its use in non-Hodgkin’s lymphoma and chronic lymphocytic leukaemia. Drugs 2003; 63(8): 803–43
Boye J, Elter T, Engert A. An overview of the current clinical use of the anti-CD20 monoclonal antibody rituximab. Ann Oncol 2003 Apr; 14(4): 520–35
Rastetter W, Molina A, White CA. Rituximab: expanding role in therapy for lymphomas and autoimmune diseases. Annu Rev Med 2004; 55: 477–503
Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma (DLBCL) in British Columbia. J Clin Oncol 2005 Aug 1; 23(22): 5027–33
Reff ME, Carner K, Chambers KS, et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood 1994; 83(2): 435–45
Ghetie M-A, Bright H, Vitetta ES. Homodimers but not monomers of rituxan (chimeric anti-CD20) induce apoptosis in human B-lymphoma cells and synergize with a chemothera-peutic agent and an immunotoxin. Blood 2001 Mar 1; 97(5): 1392–8
Demidem A, Lam T, Alas S, et al. Chimeric anti-CD20 (IDEC-C2B8) monoclonal antibody sensitizes a B cell lymphoma cell line to cell killing by cytotoxic drugs. Cancer Biother Radiopharm 1997; 12(3): 177–86
Onrust SV, Lamb HM, Barman Balfour JA. Rituximab. Drugs 1999 Jul; 58(1): 79–88
Biogen Idec Inc., Genentech Inc. Rituxan® (Rituximab): prescribing information. 2006 Feb 28
Roche Registration Limited. MabThera 100mg (concentrate for solution for infusion): summary of product characteristics [online]. Available from URL: http://www.rocheuk.com [Accessed 2006 Mar 10]
Almasri NM, Duque RE, Iturraspe J, et al. Reduced expression of CD20 antigen as a characteristic marker for chronic lymphocytic leukemia. Am J Hematol 1992; 40: 259–63
Vega MI, Huerta-Yepez S, Jazirehi AR, et al. Rituximab (chimeric anti-CD20) sensitizes B-NHL cell lines to Fas-induced apoptosis. Oncogene 2005 Aug 15; 24: 8114–27
Zhang N, Khawli LA, Hu P, et al. Generation of rituximab polymer may cause hyper-cross-linking-induced apoptosis in non-Hodgkin’s lymphomas. Clin Cancer Res 2005 Aug 15; 11(16): 5971–80
Skvortsova I, Popper BA, Skvortsov S, et al. Pretreatment with rituximab enhances radiosensitivity of non-Hodgkin’s lymphoma cells. J Radiat Res (Tokyo) 2005 Jun; 46(2): 241–8
Ghielmini M, Schmitz SF, Cogliatti SB, et al. Prolonged treatment with rituximab in patients with follicular lymphoma significantly increases event-free survival and response duration compared with the standard weekly × 4 schedule. Blood 2004 Jun 15; 103(12): 4416–23
Kennedy AD, Beum PV, Solga MD, et al. Rituximab infusion promotes rapid complement depletion and acute CD20 loss in chronic lymphocytic leukemia. J Immunol 2004 Mar 1; 172(5): 3280–8
Taylor R, Williams M, Pawluczkowycz A, et al. Thrice-weekly low dose rituximab decreases CD20 loss via shaving and suggest enhanced therapeutic targeting in chronic lymphocytic leukemia (CLL) [abstract no. 180]. Ann Oncol 2005 Jun; 16 Suppl. 5: v90
Berinstein NL, Grillo-López AJ, White CA, et al. Association of serum rituximab (IDEC-C2B8) concentration and anti-tumor response in the treatment of recurrent low-grade or follicular nonHodgkin’s lymphoma. Ann Oncol 1998 Sep; 9(9): 995–1001
McLaughlin P, Grillo-López AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol 1998 Aug; 16(8): 2825–33
Wierda WG, Keating MJ, O’Brien S. Refractory chronic lymphocytic leukemia: prognosis and treatment options. American Journal of Cancer 2004; 3(3): 163–78
Rai KR, Sawitsky A, Cronkite EP, et al. Clinical staging of chronic lymphocytic leukemia. Blood 1975 Aug; 46(2): 219–34
Dighiero G, Binet J-L. When and how to treat chronic lymphocytic leukemia. N Engl J Med 2000 Dec 14; 343(24): 1799–801
Marcus R, Imrie K, Belch A, et al. CVP chemotherapy plus rituximab compared with CVP as first-line treatment for advanced follicular lymphoma. Blood 2005 Feb 15; 105(4): 1417–23
Hiddemann W, Kneba M, Dreyling M, et al. Frontline therapy with rituximab added to the combination of cyclophos-phamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood 2005 Dec 1; 106(12): 3725–32
Forstpointner R, Dreyling M, Repp R, et al. The addition of rituximab to a combination of fludarabine, cyclophosphamide, mitoxantrone (FCM) significantly increases the response rate and prolongs survival as compared with FCM alone in patients with relapsed and refractory follicular and mantle cell lymphomas: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood 2004 Nov 15; 104(10): 3064–71
Conconi A, Martinelli G, Thiéblemont C, et al. Clinical activity of rituximab in extranodal marginal zone B-cell lymphoma of MALT type. Blood 2003 Oct 15; 102(8): 2741–5
Martinelli G, Laszlo D, Ferreri AJ, et al. Clinical activity of rituximab in gastric marginal zone non-Hodgkin’s lymphoma resistant to or not eligible for anti-Helicobacter pylori therapy. J Clin Oncol 2005 Mar 20; 23(9): 1979–83
Hainsworth JD, Litchy S, Shaffer DW, et al. Maximizing therapeutic benefit of rituximab: maintenance therapy versus retreatment at progression in patients with indolent non-Hodgkin’s lymphoma — a randomized phase II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol 2005 Feb 20; 23(6): 1088–95
Feugier P, Van Hoof A, Sebban C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol 2005 Jun 20; 23(18): 4117–26
Lenz G, Dreyling M, Hoster E, et al. Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG). J Clin Oncol 2005 Mar 20; 23(9): 1984–92
Kaplan LD, Lee JY, Ambinder RF, et al. Rituximab does not improve clinical outcome in a randomized phase III trial of CHOP with or without rituximab in patients with HIV-associated non-Hodgkin’s lymphoma: AIDS-malignancies consortium trial 010. Blood 2005 May 24
Byrd JC, Rai K, Peterson BL, et al. Addition of rituximab to fludarabine may prolong progression-free survival and overall survival in patients with previously untreated chronic lymphocytic leukemia: an updated retrospective comparative analysis of CALGB 9712 and CALGB 9011. Blood 2005 Jan 1; 105(1): 49–53
Byrd JC, Peterson BL, Morrison VA, et al. Randomized phase 2 study of fludarabine with concurrent versus sequential treatment with rituximab in symptomatic, untreated patients with B-cell chronic lymphocytic leukemia: results from Cancer and Leukemia Group B 9712 (CALGB 9712). Blood 2003 Jan 1; 101(1): 6–14
Keating MJ, O’Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol 2005 Jun 20; 23(18): 4079–88
O’Brien SM, Kantarjian HM, Cortes J, et al. Results of the fludarabine and cyclophosphamide combination regimen in chronic lymphocytic leukemia. J Clin Oncol 2001 Mar 1; 19(5): 1414–20
Wierda W, O’Brien S, Wen S, et al. Chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab for relapsed and refractory chronic lymphocytic leukemia. J Clin Oncol 2005 Jun 20; 23(18): 4070–8
Cheson BD, Horning SJ, Coiffier B., et al. Report of an International Workshop to Standardize Response Criteria for Non-Hodgkin’s Lymphomas. J Clin Oncol 1999; 17(4): 1244–53
Cheson BD, Bennett MM, Grever M, et al. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood 1996 Jun 15; 87(12): 4990–7
Ghielmini M, Schmitz SF, Cogliatti S, et al. Effect of single-agent rituximab given at the standard schedule or as prolonged treatment in patients with mantle cell lymphoma: a study of the Swiss Group for Clinical Cancer Research (SAKK). J Clin Oncol 2005 Feb 1; 23(4): 705–11
Pfreundschuh MG, Trümper L, Ma D, et al. Randomized intergroup trial of first line treatment for patients less than or equal to 60 years with diffuse large B-cell non-Hodgkin’s lymphoma (DLBCL) with a CHOP-like regimen with or without the anti-CD20 antibody rituximab — early stopping after the first interim analysis [abstract no. 6500]. 40th Annual Meeting of the American Society of Clinical Oncology; 5–8 Jun 2004; New Orleans, LA23: 556
Hainsworth JD, Litchy S, Burris HA, et al. Rituximab as first-line and maintenance therapy for patients with indolent non-Hodgkin’s lymphoma. J Clin Oncol 2002 Oct 15; 20(20): 4261–7
Habermann TM, Weller EA, Morrison VA, et al. Phase III trial of rituximab-CHOP (R-CHOP) vs. CHOP with a second randomisation to maintenance rituximab (MR) or observation in patients 60 years of age or older with diffuse large B-cell lymhoma (DLBCL) [abstract no. 8]. Blood 2003 Nov 16; 102(11): 6a
Hainsworth JD, Litchy S, Barton JH, et al. Single-agent rituximab as first-line and maintenance treatment for patients with chronic lymphocytic leukemia or small lymphocytic lymphoma: a phase II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol 2003 May 1; 21(9): 1746–51
Pfreundschuh MG, Ho A, Wolf M, et al. Treatment results of CHOP-21, CHOEP-21, MACOP-B and PMitCEBO with and without rituximab in young good-prognosis patients with aggressive lymphomas: rituximab as an ’equalizer’ in the MInT (Mabthera International Trial Group) study [abstract no. 6529 plus oral presentation]. J Clin Oncol 2005 Jun 1; 23 (Pt 1 Suppl. 16): 567
Herold M, Pasold R, Srock S, et al. Rituximab plus mitoxantrone, chlorambucil, prednisolon (R-MCP) is superior to MCP alone in advanced indolent and follicular lymphoma — results of a phase III study (OSHO39) [abstract no. 060 plus oral presentation]. Ann Oncol 2005; 16 Suppl. 5: v51–2
Solal-Celigny P, Imrie K, Belch A, et al. Mabthera (rituximab) plus CVP chemotherapy for first-line treatment of stage III/IV follicular non-Hodgkin’s lymphoma (NHL): confirmed efficacy with longer follow-up [abstract no. 350 plus oral presentation]. Blood 2005 Nov 16; 106 (11)
Salles GA, Foussard CNM, Nicolas M. Rituximab added to alFN plus CHVP improves the outcome of follicular lymphoma patients with a high tumor burden: first analysis of the GELA-GOELAMS FL-2000 randomized trial in 359 patients [abstract no. 160]. Blood 2004 Nov 16; 104 (11 Pt 1): 49–50a
Van Oers MHJ, Van Glabbeke M, Teodorovic I, et al. Chimeric anti-CD20 monoclonal antibody (Rituximab; Mabthera®) in remission induction and maintenance treatment of relapsed/resistant follicular non-Hodgkin’s lymphoma: final analysis of a phase III randomized intergroup clinical trial [abstract no. 353 plus oral presentation]. Blood 2005 Nov 16; 106 (11 Part 1): 107
Hochster HS, Weller E, Gascoyne RD, et al. Maintenance rituximab after CVP results in superior clinical outcome in advanced follicular lymphoma (FL): results of the E1496 phase III trial from the Eastern Cooperative Oncology Group and the Cancer and Leukemia Group B [abstract no. 349 plus oral presentation]. Blood 2005 Nov 16; 106 (11 Part 1): 106
Habermann TM, Weller EA, Morrison VA, et al. Rituximab-CHOP versus CHOP with or without maintenance rituximab in patients 60 years of age or older with diffuse large B-cell lymphoma (DLBCL): an update [abstract no. 127]. Blood 2004 Nov 16; 104 (11 Pt 1): 40a
Rai KR, Peterson BL, Appelbaum FR, et al. Fludarabine compared with chlorambucil as primary therapy for chronic lymphocytic leukemia. N Engl J Med 2000 Dec 14; 343(24): 1750–7
Baltazar S, Tripp G, Baez E, et al. CNOP vs. CNOP-rituximab vs. rituximab alone as first line therapy for indolent non-Hodgkin’s lymphoma (INHL): preliminary disease-free/over-all survival analysis [abstract no. 028]. 10th Congress of the European Haematology Association; 2005 Jun 2–5; Stockholm, Sweden
Dreyling MH, Forstpointner R, Ludwig W, et al. Combined immuno-chemotherapy results in superior remission rates and overall survival in recurrent follicular and mantle cell lymphoma: follow-up of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG) [abstract no. 6528 plus oral presentation]. J Clin Oncol 2005 Jun 1; 23 (Pt 1 Suppl. 16): 567
Imrie K, Belch A, Pettengell R, et al. Rituximab plus CVP chemotherapy vs CVP alone as first-line treatment for follicular lymphoma: treatment effect according to baseline prognostic factors [abstract no. 6525]. J Clin Oncol 2005 Jun 1; 23 (Pt 1 Suppl. 16): 566
Schulz H, Skoetz N, Bohlius J, et al. Does combined immu-nochemotherapy with the monoclonal antibody rituximab improve overall survival in the treatment of patients with indolent non-Hodgkin lymphoma? Preliminary results of a comprehensive meta-analysis [abstract no. 351 plus oral presentation]. Blood 2005 Nov 16; 106 (11 Part 1): 106–7
Cunningham D, Aultman R, Jost F, et al. Q-TWiST analysis of rituximab plus CVP versus CVP alone as first-line treatment for advanced follicular lymphoma [abstract no. 0685]. 10th Congress of the European Haematology Association; 2–5 Jun 2005; Stockholm, Sweden
Hiddemann W, Forstpointner R, Dreyling M, et al. Rituximab maintenance following a rituximab containing chemotherapy significantly prolongs the duration of response in patients with relapsed follicular and mantle cell lymphomas: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG) [abstract no. 6527 plus oral presentation]. J Clin Oncol 2005 Jun 1; 23 (16 Pt 1 Suppl.): 566
Van Oers MHJ, Van Glabbeke M, Teodorovic I, et al. Chimeric anti-CD20 monoclonal antibody (rituximab; Mabthera®) in remission induction and maintenance treatment of relapsed/resistant follicular non-Hodgkin’s lymphoma: a phase III randomized intergroup clinical trial [abstract no. 586]. Blood 2004 Nov 16; 104 (11 Pt 1): 169
Habermann T, Weiler E, Morrison V, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma [abstract no. 225]. Ann Oncol 2005 Jun; 16 Suppl. 5: v103
Pfreundschuh M, Kloess M, Schmits R, et al. Six, not eight cycles of bi-weekly CHOP with rituximab (R-CHOP-14) is the preferred treatment for elderly patients with diffuse large B-cell lymphoma (DLBCL): results of the RICOVER-60 trial of the German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL) [abstract no. 13 plus oral presentation]. Blood 2005 Nov 16; 106 (11)
Pfreundschuh M, Truemper L, Gill D, et al. First analysis of the completed MabThera® International (MlnT) trial in young patients with low-risk diffuse large B-cell lymphoma (DLBCL): addition of rituximab to a CHOP-like regimen significantly improves outcome of all patients with the identification of a vary favorable subgroup with IPI=0 and no bulky disease [abstract no. 157]. Blood 2004 Nov 16; 104 (11)
Gibson AD, Jain VK. Rituximab improves rate and duration of chemotherapy-induced remissions in indolent and aggressive non-Hodgkin’s lymphoma. Clinical Lymphoma 2004 Sep; 5(2): 81–3
Oriol A, Ribera JM, Lopez-Guillermo A, et al. Treatment with rituximab, CHOP and highly active antiretroviral therapy in aids-related diffuse large B-cell lymphomas: study of 48 patients [abstract no. 0784]. Haematologica 2005 Jun; 90 (Suppl. 2): 312
Rey J, Charbonnier A, Schiano de Colella JM, et al. Intensive chemotherapy with rituximab is safe and effective in AIDS non-Hodgkin’s lymphoma [letter]. AIDS 2003 Sep 5; 17(13): 2006–7
Spina M, Jaeger U, Sparano JA, et al. Rituximab plus infusional cyclophosphamide, doxorubicin, and etoposide in HIV-associated non-Hodgkin lymphoma: pooled results from 3 phase 2 trials. Blood 2005 Mar 1; 105(5): 1891–7
Kovacsovics TJ, Ketterer N, Seium Y, et al. Single-agent rituximab in active as first-line therapy in patients with newly diagnosed AIDS-related lymphoma (ARL): a pilot trial of the Swiss Groups for Clinical Cancer Research [abstract no. 0684]. 10th Congress of European Haematology Association; 2–5 Jun 2005; Stockholm, Sweden
De la Serna J, Ayala R, Canales M, et al. Treatment of diffuse large B cell lymphoma with CHOP and rituximab: results of a multicentre study with 236 patients. Blood 2005 Nov 16; 106 (11)
Trneny M, Belada D, Vasova I, et al. Rituximab combination with anthracyclin based chemotherapy significantly improved the outcome of young patients with diffuse large B-cell lymphoma in low as well in high risk subgroups [abstract no. 2444 plus poster]. Blood 2005 Nov 16; 106 (11)
Mey UJ, Strehl UJ, Orlopp KS, et al. A phase II trial of dexamethasone, high-dose cytarabine, and cisplatin (DHAP) in combination with rituximab as salvage treatment for patients with refractory or relapsed aggressive non-Hodgkin’s lymphoma [abstract no. 4618]. 46th Annual Meeting and Exposition of the American Society of Hematology; 2004 Dec 3–7; San Diego
Younes A, McLaughlin P, Romaguera J, et al. Taxol plus topotecan plus rituximab (TTR) with G-CSF support: an effective salvage program for the treatment of patients with relapsed/refractory aggressive B-cell non-Hodgkin lymphoma (NHL) who failed CHOP-like and platinum-based therapy [abstract no. 489]. Blood 2003 Nov 16; 102 Pt 1(11): 142–3
Wierda W, O’Brien S, Faderl S, et al. Improved survival in patients with relapsed — refractory chronic lymphocytic leukemia (CLL) treated with fludarabine, cyclophosphamide, and rituximab (FCR) combination [abstract no. 373]. Blood 2003 Nov 16; 102 (11 Pt 1): 110
Del Poeta G, Del Principe MI, Irno Consalvo MA, et al. The addition of rituximab to fludarabine improves clinical outcome in untreated patients with ZAP-70-negative chronic lymphocytic leukemia (in ENG]. Cancer 2005 Dec 15; 104(12): 2743–52
Hamblin T, Best JH, Hornberger J, et al. Cost-effectiveness of rituximab in treatment of diffuse large B-cell lymphoma [abstract no. 170]. Br J Haematol 2002 May; 117 Suppl. 1: 59–60
Best JH, Hornberger J, Proctor SJ, et al. Cost-effectiveness analysis of rituximab combined with CHOP for treatment of diffuse large B-cell lymphoma. Value Health Jul/Aug 2005; 8(4): 462–70
Groot MT, Lugtenburg PJ, Hornberger J, et al. Cost-effectiveness of rituximab (MabThera®) in diffuse large B-cell lymphoma in the Netherlands. Eur J Haematol 2005 Mar; 74(3): 194–202
Hornberger JC, Best JH. Cost utility in the United States of rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone for the treatment of elderly patients with diffuse large B-cell lymphoma. Cancer 2005 Apr 15; 103(8): 1644–51
Hornberger J, Lewis G. Cost-utility of rituximab (MabThera®) in diffuse large B-cell lymphoma. Eur J Cancer 2003 Sep; 1 (3 Suppl. 2): S3–7
Knight C, Hind D, Brewer N, et al. Rituximab (MabThera®) for aggressive non-Hodgkin’s lymphoma: systematic review and economic evaluation. Health Technol Assess 2004 Sep; 8(37): i–xi, 1–82
Hieke K, Pasold R, Neser S, et al. Cost evaluation of rituximab plus MCP vs. MCP alone in advanced stage indolent non-Hodgkin’s lymphoma based on a randomized controlled multicenter trial [abstract no. 87]. Blood 2004 Nov 16; 104(11 Pt 1): 28–9
Salmon K. Rituximab worth it in advanced-stage NHL? Pharmacoecon Outcomes News 2005 Feb 12; (471)
Herold M, Pasold R, Srock S, et al. Results of a prospective randomised open label phase III study comparing rituximab plus mitoxantrone, chlorambucile, prednisolone chemotherapy (R-MCP) versus MCP alone in untreated advanced indolent non-Hodgkin’s lymphoma (NHL) and mantle-cell-lymphoma (MCL). Blood 2004 Nov 16; 104(11 Pt 1): 169
Kunkel L, Wong A, Maneatis T, et al. Optimizing the use of rituximab for treatment of B-cell non-Hodgkin’s lymphoma: a benefit-risk update. Semin Oncol 2000 Dec; 27 (6 Suppl. 12): 53–61
Coiffier B, Lepage E, Brière J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 2002 Jan 24; 346(4): 235–42
Aurran-Schleinitz T, Gravis G, Vittot M, et al. “One hour” rituximab infusion is safe and improves patient care and outpatient unit management [abstract no. 4759]. Blood 2005; 106 (11)
Ghielmini M, Negretti L, Lerch E, et al. Infusion speed-escalation trial to give full-dose rituximab in one hour without steroids pre-medication [abstract no. 2451 plus poster]. Blood 2005; 106 (11)
Middleton HJ, Mollee P, Bird R, et al. Accelerated delivery of rituximab is safe on an out-patient basis [abstract no. 4777]. Blood 2005; 106 (11)
Salar A, Casao D, Pedro C, et al. Rapid infusion of rituximab with ot without steroid containing chemotherapy: a single centre experience [abstract no. 4772]. Blood 2005; 106 (11)
Sehn LH, Donaldsen J, Filewich A. Rapid infusion rituximab can be safely administered and has a positive impact on resource utilisation [abstract no. 107]. Ann Oncol 2005 Jun; 16 Suppl. 5: v69
Everington T, Goldstone AH. Novel approaches to the management of non-Hodgkin lymphoma. American Journal of Cancer 2005; 4(3): 145–58
National Comprehensive Cancer Network. Clinical practice guidelines in oncology: non-Hodgkin’s lymphoma (version 1.2005) [online]. Available from URL: http://www.nccn.org/professionals/physician_gls/PDF/nhl.pdf [Accessed 2005 Sep 5]
Pettengell R, Linch D. Position paper on the therapeutic use of rituximab in CD20-positive diffuse large B-cell non-Hodgkin’s lymphoma. Br J Haematol 2003 Apr; 121(1): 44–8
National Cancer Institute. Chronic lymphocytic leukemia (PDQ®): treatment (health professional version) [online]. Available from URL: http://www.cancer.gov/cancertopic/pdq/treatment/CLL/healthprofessional [Accessed 2005 Sep 6]
Genentech Inc. Genentech and Biogen Idec submit supplemental biologics license applpication for first-line use of Rituxan in low-grade or follicular CD20-positive B-cell non-Hodgkin’s lymphoma [media release]. 2006
Roche. MabThera maintenance therapy dramatically improves survival for patients with lymphoma [media release]. 2005
Webster K, Cella D. Quality of life in patients with low-grade non-Hodgkin’s lymphoma. Oncology 1998 May; 12(5): 697–714
Schmits R, Schmitz N, Pfreundschuh M. The best treatment for diffuse large B-cell lymphoma: a German perspective. Oncology (Huntingt) 2005 Apr; 19 (4 Suppl. 1): 16–25
Sehn LH, Connors JM. Treatment of aggressive non-Hodgkin’s lymphoma: a north American perspective. Oncology (Huntingt) 2005 Apr; 19 (4 Suppl. 1): 26–34
Coiffier B, Reyes F. Best treatment of aggressive non-Hodgkin’s lymphoma: a French perspective. Oncology (Huntingt) 2005 Apr; 19 (4 Suppl. 1): 7–15
National Cancer Institute. AIDS-related lymphoma (PDQ®): treatment (health professional version) [online]. Available from URL: http://www.cancer.gov/cancertopic/pdq/treatment/AIDS-related-lymphoma/healthprofessional [Accessed 2005 Sep 7]
Earle CC, Chapman RH, Baker CS, et al. Systemic overview of cost-utility assessments in oncology. J Clin Oncol 2000 Sep 15; 18(18): 3302–17
Petranovic D, Sever-Prebilic M, Duletic-Nacinovic A, et al. Cognitive decline in patients with non-Hodgkin lymphoma during CHOP therapy versus R-CHOP [abstract no. 1248]. 10th Congress of European Haematology Association; 2–5 Jun 2005; Stockholm, Sweden
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: J.H. Best, Department of Pharmacy, University of Washington, Seattle, Washington, USA; A. Engert, Department of Internal Medicine I, University Hospital of Cologne, Köln, Germany; M. Fanale, Department of Lymphoma/Myeloma, The University of Texas M.D. Anderson Cancer Center, Houston, Texas, USA; P. Feugier, Hematology Department, Centre Hospitalier Universitaire de Brabois, Vandoeuvre les Nancy, France; S. O’Brien, Leukemia Department, The University of Texas M.D. Anderson Cancer Center, Houston, Texas, USA; M. Pfreundschuh, Med. Klinik I, Saarland University, Homburg/Saar, Germany; P.L. Zinzani, Istituto di Ematologia e Oncologia Medica ‘L. e A. Seràgnoli’, University of Bologna, Bologna, Italy.
Data Selection
Sources: Medical literature published in any language since 1980 on ‘rituximab’, identified using MEDLINE and EMBASE, supplemented by AdisBase (a proprietary database of Adis International). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: MEDLINE search terms were ‘rituximab’ or ’MabThera’ or ‘monoclonal-antibody-IDEC-C2B8’ or ‘anti-CD20 antibodies’ and ‘non-Hodgkin’s lymphoma’ or ‘lymphoma non-Hodgkin’s’ or ‘chronic lymphocytic leukaemia’. EMBASE search terms were ‘rituximab’ or ‘MabThera’ or ‘monoclonal-antibody-IDEC-C2B8’ and ‘non-Hodgkin’s lymphoma’ or ‘chronic lymphocytic leukaemia/leukaemia’. AdisBase search terms were ‘rituximab’ or ‘monoclonal-antibody IDEC-C2B8’ or ‘MabThera’ or ‘anti-CD20 antibodies’ or ‘IDEC-C2B8’ and ‘non-Hodgkin’s lymphoma’ or ‘chronic-lymphocytic-leukaemia’ or ‘chronic lymphatic leukaemia’. Searches were last updated on 6 April 2006.
Selection: Studies in patients with who received rituximab. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Rituximab, B-cell, T-cell, lymphoma, non-Hodgkin’s lymphoma (NHL), indolent, aggressive, follicular, mantle cell, mucosa-associated lymphoid tissue (MALT), chronic lymphocytic leukaemia (CLL), pharmacodynamics, pharmacokinetics, pharmacoeconomics, therapeutic use, cost effectiveness, induction, tolerability.
Rights and permissions
About this article
Cite this article
Cvetković, R.S., Perry, C.M. Rituximab. Drugs 66, 791–820 (2006). https://doi.org/10.2165/00003495-200666060-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200666060-00005