-
PDF
- Split View
-
Views
-
Cite
Cite
Dolores Helena Rodriguez Ferreira Rivero, Sandra Regina Castro Soares, Geraldo Lorenzi-Filho, Mitiko Saiki, John J. Godleski, Leila Antonangelo, Marisa Dolhnikoff, Paulo Hilário Nascimento Saldiva, Acute Cardiopulmonary Alterations Induced by Fine Particulate Matter of São Paulo, Brazil, Toxicological Sciences, Volume 85, Issue 2, June 2005, Pages 898–905, https://doi.org/10.1093/toxsci/kfi137
Close - Share Icon Share
Abstract
The mechanisms involved in the association between air pollution and increased cardiovascular morbidity are not fully understood. The objective of this study was to test the hypothesis that fine particulate matter (PM2.5) induces systemic inflammation and vasoconstriction of small arteries in the lung and heart of rats. Thirty-eight healthy Wistar rats were anesthetized, intubated, and submitted to the instillation of 1 ml of distilled water diluted in the following solution: blank filter, 100 μg and 500 μg of PM2.5. PM2.5 was collected in glass fiber filters with a high-volume sampler. The animals were sacrificed 24 h after instillation when blood, heart, and lung samples were collected for morphological and wet-to-dry weight ratio analysis. PM2.5 consisted of the following elements: sulphur, arsenic, bromine, chlorine, cobalt, iron, lanthanum, manganese, antimony, scandium, and thorium. Total reticulocytes significantly increased at both PM2.5 doses (p < 0.05) while hematocrit levels increased in the 500 μg group (p < 0.05). Quantification of segmented neutrophils and fibrinogen levels showed a significant decrease, while lymphocytes counting increased with 100 μg of PM2.5 (p < 0.05). A significant dose-dependent decrease of intra-acinar pulmonary arteriole lumen/wall ratio (L/W) was observed in PM groups (p < 0.001). Peribronchiolar arterioles L/W showed a significant decrease in the 500 μg group (p < 0.001). A significant increase in heart wet-to-dry weight ratio was observed in the 500 μg group (p < 0.001). In conclusion, fine environment particles in the city of São Paulo promote pulmonary and cardiac histological alterations. Pulmonary vasculature was markedly affected by particle instillation, resulting in significant vasoconstriction in healthy rats.
INTRODUCTION
Recent studies have demonstrated the association between air pollution and increased cardiovascular morbidity (Lin et al., 2003; Mann et al., 2002) and mortality (Braga et al., 2001; Dockery, 2001; Goldberg et al., 2001; Schwartz et al., 2001). Because fine particulate matter (PM2.5) can access alveolar territory, components leached from these respirable particles can enter the circulation and influence important circulatory parameters that are risk factors for adverse cardiovascular events (Schwartz, 2001).
Stone and Godleski (1999) have suggested that fine particles can enter the bloodstream and adversely affect the heart by initiating arrhythmias and sudden death in susceptible subjects. More recently, it has been demonstrated that inhaled ultrafine particles can be detected within 1 min of exposure in the systemic circulation, where they can persist for hours, providing an entrance route into all organ systems (Nemmar et al., 2002). Increased plasma viscosity and changes in blood parameters such as fibrinogen levels or red blood cell counts have been demonstrated after particle inhalation (Baskurt et al., 1990; Gardner et al., 2000; Kodavanti et al., 2002; Seaton et al., 1999). A previous study carried out at our laboratory demonstrated that the composition of fine particles surrogate was critical in determining the blood response to inhaled particles (Medeiros et al., 2004). Recent studies have shown that exposure to particles induces vasoconstriction and changes in blood homeostasis favoring blood coagulation (Bouthillier et al., 1998; Peters et al., 1997). In addition, rats with acute myocardial infarction are more prone to developing ventricular arrhythmias when exposed to inhaled particles (Wellenius et al., 2002).
Cardiovascular effects of particles are not necessarily mediated only by systemic inflammation. For instance, it is plausible that the onset of pulmonary inflammation by particles (Kodavanti et al., 1998, 2002; Saldiva et al., 2002; Schins et al., 2004) may trigger reflexes that may affect cardiovascular function. Several cardiovascular effects have been documented in response to particle exposure, including disruption of the autonomic nervous system activity by increased (Magari et al., 2002; Tarkiainen et al., 2003) and decreased heart rate variability (Gold et al., 2000; Magari et al., 2001; Pope et al., 1999, 2004). Thus different mechanisms, either dependent on systemic inflammation or regulated by local reflexes, may participate in the pathogenesis of cardiovascular abnormalities associated with particle inhalation.
There is recent evidence that particle inhalation increases the generation of reactive oxidative species (ROS) in the lungs as well as in the heart (Gurgueira et al., 2002; González-Flecha, 2004; Tao et al., 2003). Production of ROS in the cardiovascular territory may be induced by several events, including inflammation (Ichimura et al., 2003; Touyz and Schiffrin, 2004), changes of autonomic control (Campese et al., 2004), or increased mechanical load (McDonough, 1999). In fact, all those pathways are interconnected and, in most of the cases, it is difficult to isolate the participation of each when evaluating particle toxicology.
The present study was designed to evaluate whether the tracheal instillation of environment particles with an aerodynamic diameter equal or lower than 2.5 μm, at two different concentrations, induces pulmonary, cardiac, and systemic inflammation in healthy adult rats. In addition, we investigated the effects morphologic parameters of the lung and heart small arteries. We reasoned that such information could contribute to the understanding of the mechanisms relating particle inhalation to acute mortality in humans.
METHODS
Animals.
Thirty-eight male adult, Wistar rats (3 months of age) weighing ∼250 g were obtained from the vivarium of our School of Medicine at the University of São Paulo. They were kept at 22° to 23°C, with controlled humidity, and a dark–light cycle of 12 to 12 h. Food and water were available ad libitum.
Particle sampling and analysis.
We employed a high-volume sampler (HIVOL, Energética, Brazil) coupled to an inlet (Tisch Environmental Inc, USA) that allows the separation of particles below 2.5 μm (PM2.5) at a flow rate of 1.1 m3/min. Our particle sampler was located on the roof of our Medical School (about 15 m above the ground), which is located on a street with very heavy traffic in downtown São Paulo. Particles were collected in one glass fiber filter, which was dried for 24 h at 50°C before and after particle collection for weighing. Particles were sampled during the month of September 2003.
Trace element determinations of the material collected were carried out on filters by neutron activation analysis. Filter samples and elemental standards were irradiated under thermal neutron flux of the IEA-R1 nuclear research reactor for 0.5 min and 16 h. After adequate decay times, the irradiated samples and standards were measured using a hyper pure Ge detector coupled with a multichannel analyzer. Element concentrations were calculated by a comparative method. Blank filters were analyzed using the same experimental conditions adopted for the analysis of filter samples, and the concentrations of the measured elements in the blank filter were subtracted to provide their net concentration. Contribution from the blank filter was also subtracted from results, and the final results were then expressed as a function of collected air volume. Sulfur determination was performed by x-ray fluorescence analysis, using the non-exposed part of the filters as a blank filter.
Filter extracts for tracheal instillation.
Filter extracts were prepared within 24 h after collection. Because of the inherent difficulties in removing particulate material directly from a high-volume filter, subcomponents of PM were extracted in distilled water via agitation by ultrasonication water bath for 8 h. The efficiency of extraction was determined by weighting the filter before and after extraction, and it was determined as 79%. The weight of the filters was determined after drying them for 24 h at 50°C.
Tracheal instillation.
The rats were anaesthetized with 3% sodium pentobarbital (30 mg/kg body weight, i.p.) and submitted to tracheal intubation with an adapted pediatric laryngoscope; a 16-gauge polyethylene tube was inserted, working as an endotracheal tube. Test solutions (1 ml) were slowly injected in three separate inspirations through the endotracheal tube coupled to a syringe. Animals were allowed to recover between instillations, because a reflex apnea was induced each time. The rats were randomized and received the following solutions:The entire group of 38 animals was submitted to the analysis.
BF—blank filter (n = 12): solution obtained by ultra-sonication of a blank filter in distilled water;
PM100 (n = 13): solution obtained by ultrasonication of a filter containing PM submerged in distilled water, corresponding to 100 μg of PM2.5;
PM500 (n = 13): solution obtained by ultra-sonication of a filter containing PM submerged in distilled water (the same procedure as PM100) corresponding to 500 μg of PM2.5.
Blood analysis.
Rats were anaesthetized with sodium pentobarbital (50 mg/kg body weight, i.p.) 24 h after tracheal instillation. Blood samples were collected by abdominal aorta puncture, and blood was stored in ethylene diamine tetraacetic acid (EDTA) K3 tubes for complete blood and reticulocyte count. Complete blood counts of red blood cells, platelets, and white blood cells were performed using a hematological analyzer (Bayer Corporation, NY). For the determination of the reticulocytes, the whole blood was diluted with a fluorescent stain (thiazole orange), which is specific for nucleic acids (Corberand, 1996). After 25 s, solution fluorescence was measured with a laser optical bench. The equipment gives the percentage of total reticulocytes counted. In addition, blood samples were collected in vials with sodium citrate for the determination of fibrinogen, activated partial thromboplastin time, and prothrombin time.
Histopathology and morphometry.
The heart was isolated en bloc and fixed for 48 h in buffered 10% formalin solution. Before the heart was fixed in formalin, one transverse section was sampled for wet-to-dry weight ratio determination. After fixation, the heart was cut in three parallel transverse sections of both ventricular chambers, 2 mm apart.
The left lung was isolated and fixed for 24 h by intratracheal instillation of 10% formalin solution at a constant pressure of 20 cm of water. A transverse section was performed at the entrance of the main left bronchus. Both heart and lung tissue slices were embedded in paraffin and processed according to routine histological procedures. Five-micrometer thick slides were prepared and stained with hematoxylin and eosin (H&E).
Histological slices were coded for blind analysis. Descriptive histopathology was performed for all sections sampled. Quantitative measurements of the ratio between the lumen and wall (L/W) areas were done for transverse sections of coronary arteries. Lung L/W ratio was measured in transversally cut arterioles adjacent to the terminal bronchioles, located in the transition between those conducting to gas exchange airways and in intra-acinar arterioles. Transversally cut vessels were defined as the ones exhibiting a variation between its maximum and minimum diameter ≤10%. Morphometric measurements were performed using a standard point-counting procedure, at 400×, with the aid of a coherent test system of 100 points and 50 lines contained within a square of 10,000 μm2 at this magnification. Arterial diameters were assessed using the grid lines. L/W ratio was determined by placing the entire artery within the limits of the square, and determining the number of points hitting the lumen or the wall of the vessel. The areas of arteriolar lumen and wall were then calculated according to equations (1) and (2):After measurements, pulmonary and myocardial arterioles were categorized by size, according to the luminal area.
(1) luminal area = number of points hitting the lumen × 100
(2) wall area = number of points hitting the arteriolar wall × 100
Wet-to-dry weight ratio.
The severity of pulmonary and heart edema was assessed by the wet-to-dry weight ratio. The entire inferior right lobe of the lung and one transverse slice of the heart, comprising the right and left ventricles, were gently placed in a pre-weighted Petri dish, and weighted on an analytical balance immediately after excision to obtain the wet weight. Each specimen was then dried at 50°C for 72 h to obtain the dry weight. The wet-to-dry weight ratio was calculated as follows:
(3) wet-to-dry weight ratio = (wet weight − dry weight)/wet weight.
Statistical analysis.
For the morphometric data of pulmonary and coronary vessels, we tested the significance of the results by computing general linear models, using as dependent variable either the absolute value or ranks of lumen/wall ratio. As predictive variables, we considered categorical terms for treatment (blank filter, 100 and 500 μg of PM2.5) and three indicators of the size of the vessel evaluated, as well as a term of interaction between treatment and size. The indicator term for vessel size was included because lumen/wall ratio is expected to increase as the lumen of the vessel increases. For hematological parameters, the significance of results was determined using analysis of variance (ANOVA) for independent groups, setting the level of significance at 5%. Since there was a significant degree of heterocedasticity in several variables analyzed, we again considered as dependent variables in the ANOVA models either the absolute value or ranks of each parameter measured. Bonferroni post-hoc analysis was used when significant statistical differences were detected by general linear models or ANOVA. The statistical package employed was the SPSS v. 10.0 for Windows.
RESULTS
Particle Analysis
Table 1 shows the values of element composition measured by neutron activation analysis and, in the case of sulfur, x-ray fluorescence analysis. In general, the concentrations of sulfur, bromide, cobalt, and manganese of the air in São Paulo are higher than those reported for other locations such as Los Angeles, New Jersey, Virginia, Boston, and Phoenix, with the exception of iron (Harrison and Yin, 2000; Prahalad et al., 1999).
Elemental Composition and Concentration of PM2.5 Particles
Element (%) . | Values . |
|---|---|
| Sulfura | 3.05 |
| Arsenic | 0.30 |
| Bromine | 0.21 |
| Chorine | 2.09 |
| Cobalt | 2.65 |
| Iron | 2.67 |
| Lanthanum | 5.42 |
| Manganese | 0.64 |
| Antimony | 0.21 |
| Scandium | 3.25 |
| Thorium | 8.14 |
Element (%) . | Values . |
|---|---|
| Sulfura | 3.05 |
| Arsenic | 0.30 |
| Bromine | 0.21 |
| Chorine | 2.09 |
| Cobalt | 2.65 |
| Iron | 2.67 |
| Lanthanum | 5.42 |
| Manganese | 0.64 |
| Antimony | 0.21 |
| Scandium | 3.25 |
| Thorium | 8.14 |
X-ray fluorescence analysis.
Elemental Composition and Concentration of PM2.5 Particles
Element (%) . | Values . |
|---|---|
| Sulfura | 3.05 |
| Arsenic | 0.30 |
| Bromine | 0.21 |
| Chorine | 2.09 |
| Cobalt | 2.65 |
| Iron | 2.67 |
| Lanthanum | 5.42 |
| Manganese | 0.64 |
| Antimony | 0.21 |
| Scandium | 3.25 |
| Thorium | 8.14 |
Element (%) . | Values . |
|---|---|
| Sulfura | 3.05 |
| Arsenic | 0.30 |
| Bromine | 0.21 |
| Chorine | 2.09 |
| Cobalt | 2.65 |
| Iron | 2.67 |
| Lanthanum | 5.42 |
| Manganese | 0.64 |
| Antimony | 0.21 |
| Scandium | 3.25 |
| Thorium | 8.14 |
X-ray fluorescence analysis.
Blood Analysis
Table 2 shows the descriptive statistics of hematological parameters. There was a significant increase in total reticulocytes at both doses of PM2.5 (p < 0.05) while hematocrit levels increased for the group receiving 500 μg in relation to the blank filter group (p = 0.04). Segmented and neutrophils showed a significant decrease in the 100 μg group in relation to the group receiving 500 μg of PM2.5 (p = 0.03). Lymphocytes showed a significant increase at the 100 μg concentration in relation to 500μg of PM2.5 (p = 0.02). The remaining parameters did not exhibit significant alterations. No significant differences were observed among the groups regarding platelet counting, prothrombin time, and activated partial thromboplastin time. There was a significant decrease in fibrinogen levels at the dose of 100 μg in relation to the blank filter (p = 0.01).
Descriptive Statistics (Mean, Median and Standard Deviation) of Blood Cells and Coagulation Factors in Circulating Blood from Animals Exposed to Blank Filter, 100 μg, and 500 μg of PM2.5
. | Group . | . | . | . | . | . | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Blank filter . | . | . | PM100 . | . | . | PM500 . | . | . | ||||||||
. | Mean . | Median . | S.D. . | Mean . | Median . | S.D. . | Mean . | Median . | S.D. . | ||||||||
| Erytrocytes (million/mm3) | 7.88 | 7.86 | .50 | 8.01 | 8.03 | .41 | 8.10 | 8.06 | .50 | ||||||||
| Hematocrit (%)a | 40.69 | 40.90 | 2.00 | 42.76 | 43.80 | 2.64 | 43.62 | 43.90 | 1.77 | ||||||||
| Segmented (%)b | 19.17 | 15.50 | 14.81 | 14.15 | 10.00 | 10.94 | 27.00 | 22.00 | 14.97 | ||||||||
| Band (%) | 1.54 | 1.00 | .99 | 1.62 | 1.00 | 1.66 | 1.08 | 1.00 | .28 | ||||||||
| Lymphocytes (%)b | 72.75 | 77.50 | 14.11 | 79.62 | 84.00 | 11.89 | 67.31 | 69.00 | 14.21 | ||||||||
| Monocytes (%) | 3.25 | 3.00 | 1.96 | 3.23 | 3.00 | 2.45 | 3.77 | 3.00 | 3.11 | ||||||||
| Eosinophils (%) | .67 | .50 | .78 | .69 | 1.00 | .63 | .85 | .00 | 1.14 | ||||||||
| Basophils (%) | .00 | .00 | .00 | .00 | .00 | .00 | .00 | .00 | .00 | ||||||||
| Neutrophils (%)b | 23.33 | 19.00 | 14.30 | 16.46 | 12.00 | 9.82 | 28.08 | 23.00 | 15.16 | ||||||||
| Reticulocytes (%)c | 3.25 | 3.20 | .46 | 3.73 | 3.70 | .71 | 3.93 | 3.80 | .93 | ||||||||
| Platelets (thousand/mm3) | 833.9 | 831.00 | 132.29 | 799.0 | 805.00 | 98.96 | 878.7 | 925.00 | 216.08 | ||||||||
| Fibrinogend (mg/dl) | 397.5 | 385.50 | 39.40 | 353.8 | 345.50 | 48.94 | 386.2 | 383.00 | 36.76 | ||||||||
| Prothrombin time (s) | 23.55 | 23.10 | 1.99 | 24.40 | 24.10 | 1.41 | 24.58 | 24.80 | 1.35 | ||||||||
| Active partial thromboplastin time (s) | 30.50 | 24.00 | 10.76 | 27.35 | 24.00 | 8.19 | 34.16 | 30.15 | 11.58 | ||||||||
. | Group . | . | . | . | . | . | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Blank filter . | . | . | PM100 . | . | . | PM500 . | . | . | ||||||||
. | Mean . | Median . | S.D. . | Mean . | Median . | S.D. . | Mean . | Median . | S.D. . | ||||||||
| Erytrocytes (million/mm3) | 7.88 | 7.86 | .50 | 8.01 | 8.03 | .41 | 8.10 | 8.06 | .50 | ||||||||
| Hematocrit (%)a | 40.69 | 40.90 | 2.00 | 42.76 | 43.80 | 2.64 | 43.62 | 43.90 | 1.77 | ||||||||
| Segmented (%)b | 19.17 | 15.50 | 14.81 | 14.15 | 10.00 | 10.94 | 27.00 | 22.00 | 14.97 | ||||||||
| Band (%) | 1.54 | 1.00 | .99 | 1.62 | 1.00 | 1.66 | 1.08 | 1.00 | .28 | ||||||||
| Lymphocytes (%)b | 72.75 | 77.50 | 14.11 | 79.62 | 84.00 | 11.89 | 67.31 | 69.00 | 14.21 | ||||||||
| Monocytes (%) | 3.25 | 3.00 | 1.96 | 3.23 | 3.00 | 2.45 | 3.77 | 3.00 | 3.11 | ||||||||
| Eosinophils (%) | .67 | .50 | .78 | .69 | 1.00 | .63 | .85 | .00 | 1.14 | ||||||||
| Basophils (%) | .00 | .00 | .00 | .00 | .00 | .00 | .00 | .00 | .00 | ||||||||
| Neutrophils (%)b | 23.33 | 19.00 | 14.30 | 16.46 | 12.00 | 9.82 | 28.08 | 23.00 | 15.16 | ||||||||
| Reticulocytes (%)c | 3.25 | 3.20 | .46 | 3.73 | 3.70 | .71 | 3.93 | 3.80 | .93 | ||||||||
| Platelets (thousand/mm3) | 833.9 | 831.00 | 132.29 | 799.0 | 805.00 | 98.96 | 878.7 | 925.00 | 216.08 | ||||||||
| Fibrinogend (mg/dl) | 397.5 | 385.50 | 39.40 | 353.8 | 345.50 | 48.94 | 386.2 | 383.00 | 36.76 | ||||||||
| Prothrombin time (s) | 23.55 | 23.10 | 1.99 | 24.40 | 24.10 | 1.41 | 24.58 | 24.80 | 1.35 | ||||||||
| Active partial thromboplastin time (s) | 30.50 | 24.00 | 10.76 | 27.35 | 24.00 | 8.19 | 34.16 | 30.15 | 11.58 | ||||||||
BF different from PM500 (p = 0.04).
PM100 different from PM500 (p < 0.05).
BF different from both doses of PM (p < 0.05).
PM100 different from BF (p = 0.01).
Descriptive Statistics (Mean, Median and Standard Deviation) of Blood Cells and Coagulation Factors in Circulating Blood from Animals Exposed to Blank Filter, 100 μg, and 500 μg of PM2.5
. | Group . | . | . | . | . | . | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Blank filter . | . | . | PM100 . | . | . | PM500 . | . | . | ||||||||
. | Mean . | Median . | S.D. . | Mean . | Median . | S.D. . | Mean . | Median . | S.D. . | ||||||||
| Erytrocytes (million/mm3) | 7.88 | 7.86 | .50 | 8.01 | 8.03 | .41 | 8.10 | 8.06 | .50 | ||||||||
| Hematocrit (%)a | 40.69 | 40.90 | 2.00 | 42.76 | 43.80 | 2.64 | 43.62 | 43.90 | 1.77 | ||||||||
| Segmented (%)b | 19.17 | 15.50 | 14.81 | 14.15 | 10.00 | 10.94 | 27.00 | 22.00 | 14.97 | ||||||||
| Band (%) | 1.54 | 1.00 | .99 | 1.62 | 1.00 | 1.66 | 1.08 | 1.00 | .28 | ||||||||
| Lymphocytes (%)b | 72.75 | 77.50 | 14.11 | 79.62 | 84.00 | 11.89 | 67.31 | 69.00 | 14.21 | ||||||||
| Monocytes (%) | 3.25 | 3.00 | 1.96 | 3.23 | 3.00 | 2.45 | 3.77 | 3.00 | 3.11 | ||||||||
| Eosinophils (%) | .67 | .50 | .78 | .69 | 1.00 | .63 | .85 | .00 | 1.14 | ||||||||
| Basophils (%) | .00 | .00 | .00 | .00 | .00 | .00 | .00 | .00 | .00 | ||||||||
| Neutrophils (%)b | 23.33 | 19.00 | 14.30 | 16.46 | 12.00 | 9.82 | 28.08 | 23.00 | 15.16 | ||||||||
| Reticulocytes (%)c | 3.25 | 3.20 | .46 | 3.73 | 3.70 | .71 | 3.93 | 3.80 | .93 | ||||||||
| Platelets (thousand/mm3) | 833.9 | 831.00 | 132.29 | 799.0 | 805.00 | 98.96 | 878.7 | 925.00 | 216.08 | ||||||||
| Fibrinogend (mg/dl) | 397.5 | 385.50 | 39.40 | 353.8 | 345.50 | 48.94 | 386.2 | 383.00 | 36.76 | ||||||||
| Prothrombin time (s) | 23.55 | 23.10 | 1.99 | 24.40 | 24.10 | 1.41 | 24.58 | 24.80 | 1.35 | ||||||||
| Active partial thromboplastin time (s) | 30.50 | 24.00 | 10.76 | 27.35 | 24.00 | 8.19 | 34.16 | 30.15 | 11.58 | ||||||||
. | Group . | . | . | . | . | . | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Blank filter . | . | . | PM100 . | . | . | PM500 . | . | . | ||||||||
. | Mean . | Median . | S.D. . | Mean . | Median . | S.D. . | Mean . | Median . | S.D. . | ||||||||
| Erytrocytes (million/mm3) | 7.88 | 7.86 | .50 | 8.01 | 8.03 | .41 | 8.10 | 8.06 | .50 | ||||||||
| Hematocrit (%)a | 40.69 | 40.90 | 2.00 | 42.76 | 43.80 | 2.64 | 43.62 | 43.90 | 1.77 | ||||||||
| Segmented (%)b | 19.17 | 15.50 | 14.81 | 14.15 | 10.00 | 10.94 | 27.00 | 22.00 | 14.97 | ||||||||
| Band (%) | 1.54 | 1.00 | .99 | 1.62 | 1.00 | 1.66 | 1.08 | 1.00 | .28 | ||||||||
| Lymphocytes (%)b | 72.75 | 77.50 | 14.11 | 79.62 | 84.00 | 11.89 | 67.31 | 69.00 | 14.21 | ||||||||
| Monocytes (%) | 3.25 | 3.00 | 1.96 | 3.23 | 3.00 | 2.45 | 3.77 | 3.00 | 3.11 | ||||||||
| Eosinophils (%) | .67 | .50 | .78 | .69 | 1.00 | .63 | .85 | .00 | 1.14 | ||||||||
| Basophils (%) | .00 | .00 | .00 | .00 | .00 | .00 | .00 | .00 | .00 | ||||||||
| Neutrophils (%)b | 23.33 | 19.00 | 14.30 | 16.46 | 12.00 | 9.82 | 28.08 | 23.00 | 15.16 | ||||||||
| Reticulocytes (%)c | 3.25 | 3.20 | .46 | 3.73 | 3.70 | .71 | 3.93 | 3.80 | .93 | ||||||||
| Platelets (thousand/mm3) | 833.9 | 831.00 | 132.29 | 799.0 | 805.00 | 98.96 | 878.7 | 925.00 | 216.08 | ||||||||
| Fibrinogend (mg/dl) | 397.5 | 385.50 | 39.40 | 353.8 | 345.50 | 48.94 | 386.2 | 383.00 | 36.76 | ||||||||
| Prothrombin time (s) | 23.55 | 23.10 | 1.99 | 24.40 | 24.10 | 1.41 | 24.58 | 24.80 | 1.35 | ||||||||
| Active partial thromboplastin time (s) | 30.50 | 24.00 | 10.76 | 27.35 | 24.00 | 8.19 | 34.16 | 30.15 | 11.58 | ||||||||
BF different from PM500 (p = 0.04).
PM100 different from PM500 (p < 0.05).
BF different from both doses of PM (p < 0.05).
PM100 different from BF (p = 0.01).
Histopathology and Morphometry
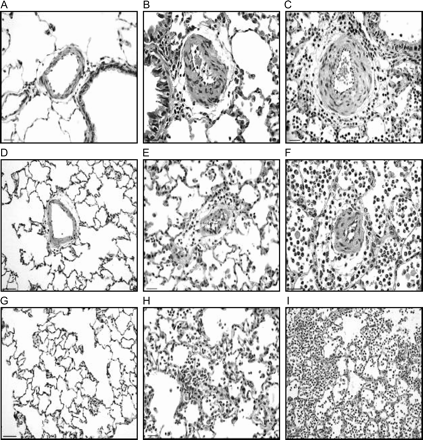
Figure 1 shows the pulmonary changes observed in the three groups. Animals submitted to PM100 and PM500 concentrations developed an acute pulmonary inflammation in the alveolar tissue, characterized by the recruitment of neutrophils and macrophages at the transition between the terminal bronchiole and gas-exchange territory with the involvement of the peripheral alveolar parenchyma. Although we did not quantify the pulmonary inflammatory changes, histological analysis showed a dose-dependent pattern of alveolar inflammation, which was more pronounced in the PM500 group. Increased numbers of inflammatory cells were also detected in the peribronchial and perivascular connective tissue in the two groups. There was no evidence of myocardial inflammation in either group.
Photomicrographs of pulmonary tissue in blank filter (A, D, and G), PM100 (B, E, and H) and PM500 (C, F, and I) groups. A to C: The figures show peribronchiolar arterioles with decreased L/W ratio in PM500 group (C), secondary to vasoconstriction. D to F: The intra-acinar arterioles show vasoconstriction and decrease in L/W ratio in both 100PM (E) and 500PM (F) groups as compared with blank filter (D). Note the periarteriolar inflammation in the PM groups. G to I: Acute alveolar inflammation is observed in both PM groups (H and I), which is more pronounced in the animals exposed to higher PM dose (I). Scale bar in A, D, G, and I = 50 μm. Scale bar in B, C, E, F, and H = 25 μm.
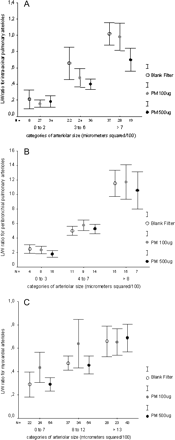
Figure 2A depicts the results of L/W ratio measured in intra-acinar pulmonary arterioles, aggregated by vessel size and exposure. A significant decrease of L/W ratio was observed in the animals exposed to environment particles (p < 0.001). In addition, the decrement of L/W ratio was different between animals receiving the higher dose (500 μg) in comparison with those receiving the smaller dose (100 μg) (p = 0.002). Figure 2B shows the L/W ratio measured in arterioles located adjacent to terminal bronchioles. The PM500 group showed a significant decrease for all arteriolar sizes in relation to the blank filter and PM100 μg (p < 0.001) groups. Pulmonary arteriolar changes are illustrated in Figure 1. Figure 2C presents the results of L/W ratio measured in myocardial arterioles. No difference was observed among the 3 treatments employed (p = 0.08).
Mean and standard error of lumen/wall ratio values in lung and heart for the three groups (blank filter, 100 μg and 500 μg of PM2.5). (A) L/W ratio of intra-acinar pulmonary arterioles was significantly decreased in PM groups (100 and 500 μg) as compared with blank filter (p < 0.001). Additionally, the L/W ratio was different between PM500 and PM100 (p = 0.002). (B) Peribronchiolar pulmonary arterioles showed significantly decreased L/W ratio in PM 500 group as compared with blank filter and PM100 (p < 0.001). (C) Lumen/wall ratio measured in myocardial arterioles. No difference was observed among the groups.
Wet-to-Dry Weight Ratio
Table 3 shows the results of the lung and heart wet-to-dry weight ratio. A significant increase of this relation was observed only in the heart, with the higher dose of PM (500 μg) being different from the remaining treatments (p < 0.001).
Mean and Standard Deviation of Wet/Dry Weight Ratio in Lung and Heart from Animals Exposed to Blank Filter, 100 μg, and 500 μg of PM2.5
. | Wet/dry weight ratio . | . | |
|---|---|---|---|
. | Lung . | heart . | |
| Blank filter | 0.81 ± 0.02 | 0.86 ± 0.03 | |
| PM100 μg | 0.81 ± 0.02 | 0.84 ± 0.05 | |
| PM500 μg | 0.81 ± 0.06 | 0.87 ± 0.04* | |
. | Wet/dry weight ratio . | . | |
|---|---|---|---|
. | Lung . | heart . | |
| Blank filter | 0.81 ± 0.02 | 0.86 ± 0.03 | |
| PM100 μg | 0.81 ± 0.02 | 0.84 ± 0.05 | |
| PM500 μg | 0.81 ± 0.06 | 0.87 ± 0.04* | |
p < 0.001 compared to blank filter and PM100 μg.
Mean and Standard Deviation of Wet/Dry Weight Ratio in Lung and Heart from Animals Exposed to Blank Filter, 100 μg, and 500 μg of PM2.5
. | Wet/dry weight ratio . | . | |
|---|---|---|---|
. | Lung . | heart . | |
| Blank filter | 0.81 ± 0.02 | 0.86 ± 0.03 | |
| PM100 μg | 0.81 ± 0.02 | 0.84 ± 0.05 | |
| PM500 μg | 0.81 ± 0.06 | 0.87 ± 0.04* | |
. | Wet/dry weight ratio . | . | |
|---|---|---|---|
. | Lung . | heart . | |
| Blank filter | 0.81 ± 0.02 | 0.86 ± 0.03 | |
| PM100 μg | 0.81 ± 0.02 | 0.84 ± 0.05 | |
| PM500 μg | 0.81 ± 0.06 | 0.87 ± 0.04* | |
p < 0.001 compared to blank filter and PM100 μg.
DISCUSSION
Our results indicated that a single instillation of urban particles elicits significant pulmonary and cardiac alterations, and, to a lesser extent, changes in blood parameters.
Lung inflammatory alterations were quite evident in the animals exposed to PM2.5 up to a point that it was possible to distinguish, without error, the histological slices of the exposed animals from controls. To avoid an interpretation bias, the researcher who conducted pulmonary vessel measurements did not perform the descriptive histopathological studies of the lungs. Our findings are in line with several previous studies and fully confirm the inflammatory potential of urban PM2.5 aerosol. Interestingly, we observed a significant narrowing of the pulmonary vasculature in the animals that were exposed to PM2.5, with this effect being more prominent in the intra-acinar arterioles. This result confirms our previous finding that the pulmonary vasculature is a target for urban particles (Batalha et al., 2002). Because of the short time lag between instillation and evaluation (24 h) the narrowing of pulmonary vasculature is most probably due to constriction rather than being dependent on significant arteriolar remodeling. Thus, our results suggest that exposure to environment particles modifies the balance between vasoconstriction and vasodilatation at pulmonary level. The endothelium is activated by circulating cytokines that induce the production of nitric oxide, reactive oxygen species and endothelin in vessel walls, which are potent vascular smooth-muscle constrictors. Bouthillier et al. (1998) showed increased plasma levels of endotelin −1 in rats after inhalation of urban particles.
The inspection of Figure 1 shows that, although a small expansion of the perivascular and peribronchial connective tissue by interstitial edema can be noticed, intra-alveolar edema is virtually absent in animals receiving PM, even at the highest dose. This finding is in agreement with the observation of a slight and non-significant increase in wet/dry ratio, as depicted in Table 3. In fact, the magnitude of inflammatory cell recruitment clearly overcomes fluid exudation to alveolar space. This is an interesting finding and, perhaps, it is related to the peculiar composition of our particles. Unfortunately, we did not have a comprehensive characterization of the chemical composition of our test particles, since we only measured 11 elements and we do not have information about the organic material adhered to the PM. Assuming that in study, the sulfur content is mainly in the form of sulfate, the levels of this component in our PM samples are not very high. Considering that our light duty vehicles uses a blend of gasoline with 22% of ethanol, it is quite probable that PM in our town contains a significant proportion of volatile organic compounds (including aldehydes) and nitrates. Indeed, the composition of the particles was shown to influence their toxicity in vivo (Kodavanti et al., 1998; Saldiva et al., 2002; Schins et al., 2004) and in vitro (Carter et al., 1997; González-Flecha, 2004; Prahalad et al., 1999). It is tempting to speculate that the characteristics of our pollution scenario determine the predominance of cellular accumulation over edema in the lungs of our animals, but the limited information about the composition of our particles prevents us from drawing mechanistic hypothesis, indicating the necessity of further studies, probably comparing our PM to samples from places with different pollution sources.
The concept that particles induce constriction of pulmonary arterioles is new and opens new possibilities for the study of cardiac impairment induced by particles since, conceivably, constriction of pulmonary vasculature induces an increase of the mechanical load to the heart. In this line, recent epidemiological data indicate that patients with congestive heart failure are among those with the highest susceptibility to particle pollution exposure (Mann et al., 2002). In light of our findings, the possibility that an increased afterload to the right ventricle induced by particles may further deteriorate cardiac performance in subjects with congestive heart failure (CHF) must be considered as one of the mechanisms correlating air pollution and cardiac diseases. It is also important to stress that any increment in the mechanical load to the heart induces an increase in oxygen consumption by the cardiac cells, predisposing to the development of cardiac hypoxia in situations where the oxygen available to cardiomyocytes is already insufficient. Myocardial hypoxia is an event that predisposes to both myocardial infarction (Peters et al., 2001) and arrhythmias (Peters et al., 2000; Watkinson et al., 1998), events that have been related to particle pollution by the epidemiology literature.
Despite the significant pulmonary inflammation observed, the changes in blood markers evaluated in our study show a somehow lower intensity. We detected a decrease in fibrinogen and neutrophils in the rats exposed to the low dose of PM2.5. This finding is somewhat puzzling. One explanation could be that our lower dose of particles induced a transient adherence of neutrophils to the microvasculature, as well as a consumption of fibrinogen, changes that were reversed at the higher doses, with release of neutrophils and fibrinogen by the bone marrow and liver, respectively. In this respect, the hematological changes observed in our study are less intense than those reported in a previous study of our group in animals exposed to residual oil fly ash (Medeiros et al., 2004). It is possible that our PM2.5 contains substances with a lower solubility and capacity to reach the systemic circulation. It is remarkable that reticulocytes were increased after particle instillation. Previous studies (Goto et al., 2004; Medeiros et al., 2004; Van Eeden and Hogg, 2002) have also observed that bone marrow is responsive to inhaled particles. The increased number of reticulocytes may induce changes in blood viscosity, which may contribute to mechanical load to the heart, as well as to blood clotting. Thus, our results suggest that, although modest in our case, changes in blood parameters induced by particle inhalation may participate in the pathogenesis of congestive heart failure aggravation and myocardial ischemic diseases reported as associated with particle inhalation by epidemiological studies.
Cardiac edema was also observed in animals receiving PM2.5. The lungs did not exhibit such alteration, suggesting that the lymphatic drainage in the lungs is an efficient mechanism. Cardiac edema may occur as a consequence of several physiological and pathological conditions, and the design of our study did not allow the clarification of its pathogenesis. However, the presence of cardiac edema, measured 24 h after particle instillation, is in line with the hypothesis that environment particles do affect heart functioning.
In conclusion, our results indicate that exposure to environment particles promotes pulmonary and cardiac alterations in healthy rats, even when cellular markers of systemic inflammation are not evidently altered. Pulmonary vasculature was also markedly affected by particle instillation, resulting in significant vasoconstriction. In addition, we observed that the blood marrow also participated in the acute response to particles reaching the lungs. The overall results supportive the conclusion that environmental particles affect not only the lungs but also the heart and suggest that these changes may occur even when modest alterations of blood cell markers of systemic inflammation are observed.
References
Baskurt, O. K., Levi, E., Caglayan, S., and Dikmenoglu, N. (
Batalha, J. R. F., Saldiva, P. H. N., Clarke, R. W., Coull, B. A., Stearns, R. C., Lawrence, J., Murthy, G. G. K., Koutrakis, P., and Godleski, J. J. (
Bouthillier, L., Vincent, R., Goegan, P., Adamson, I. Y. R., Bjarnason, S., Stewart, M., Guénette, J., Potvin, M., and Kumarathasan, P. (
Braga, A. L. F., Zanobetti, A., and Schwartz, J. (
Campese, V. M., Ye, S., Zhong, H., Yanamadala, V., Ye, Z., and Chiu, J. (
Carter, J. D., Ghio, A. J., Samet, J. M., and Devlin, R. B. (
Corberand, J. X. (
Dockery, D. W. (
Gardner, S. Y., Lehmann, J. R., and Costa, D. L. (
Gold, D. R., Litonjua, A., Schwartz, J., Lovett, E., Larson, A., Nearing, B., Allen, G., Verrier, M., Cherry, R., and Verrier, R. (
Goldberg, M. S., Burnett, R. T., Bailar, J. C. 3rd, Tamblyn, R., Ernst, P., Flegel, K., Brook, J., Bonvalot, Y., Singh, R., Valois, M. F., and Vincent, R. (
González-Flecha, B. (
Goto, Y., Ishii, H., Hogg, J. C., Shih, C. H., Yatera, K., Vincent, R., and Van Eeden, S. F. (
Gurgueira, S. A., Lawrence, J., Coull, B., Murthy, G. G. K. and González-Flecha, B. (
Harrison, R. M., and Yin, J. (
Ichimura, H., Parthasarathi, K., Quadri, S., Issekutz, A. C., and Bhattacharya, J. (
Kodavanti, U. P., Hauser, R., Christiani, D. C., Zhi, H. M., McGee, J., Ledbetter, A., Richards, J., and Costa, D. L. (
Kodavanti, U. P., Schladweiler, M. C., and Ledbetter, A. D. (
Lin, C. A., Pereira, L. A. A., Conceição, G. M. S., Kishi, H. S., Milani, R. Jr., Braga, A. L. F., and Saldiva, P. H. N. (
Magari, S. R., Hauser, R., Schwartz, J., Williams, P. L., Smith, T. J., and Christiani, D. C. (
Magari, S. R., Schwartz, J., Williams, P. L., Hauser, R., Smith, T. J., and Christiani, D. C.
McDonough, K. H. (
Mann, J. K., Tager, I. B., Lurmann, F., Segal, M., Quesenberry, C. P., Lugg, M. M., Shan, J., and Van Den Eeden, S. K. (
Medeiros, N., Jr., Rivero, D. H. R. F., Kasahara, D. I., Saiki, M., Godleski, J. J., Koutrakis, P., Capelozzi, V. L., Saldiva, P. H. N., and Antonangelo, L. (
Nemmar, A., Hoet, P. H. M., Vanquickenborne, B., Dinsdale, D., Thomeer, M., Hoylaerts, M. F., Vanbilloen, H., Mortelmans, L. and Nemery, B. (
Peters, A., Döring, A., Wichmann, H-E., and Koenig, W. (
Peters, A., Liu, E., Verrier, R. L., Schwartz, J., Gold, D. R., Mittleman, M., Baliff, J., Oh, J. A., Allen, G., Monahan, K., and Dockery, D. W. (
Peters, A., Dockery, D. W., Muller, J. E., and Mittleman, M. A. (
Pope, C. A. 3rd, Verrier, R. L., Lovett, E. G., Larson, A. C., Raizenne, M. E. B. A., Kanner, R. E., Schwartz., J., Villegas, G. M., Gold, D. R., and Dockery, D. W. (
Pope, C. A. 3rd, Hansen, M. L., Long, R. W., Nielsen, K. R., Eatough, N. L., Wilson, W. E., and Eatough, D. J. (
Prahalad, A. K., Soukup, J. M., Inmon, J., Willis, R., Ghio, A. J., Becker, S., and Gallagher, J. E. (
Saldiva, P. H. N., Clarke, R. W., Coull, B. A., Stearns, R. C., Murthy, J. L. G. G., Diaz, E., Koutrakis, P., Suh, H., Tsuda, A., and Godleski, J. J. (
Schins, R. P. F., Lightbody, J. H., Borm, P. J. A., Shi, T., Donaldson, K., and Stone, V. (
Schwartz, J. (
Schwartz, J., Ballester, F., Saez, M., Perez-Hoyos, S., Bellido, J., Cambra, K., Arribas, F., Cañada, A., Perez-Boillos, M. J., and Sunyer, J. (
Seaton, A., Soutar, A., Crawford, V., Elton, R., McNerlan, S., Cherrie, J., Watt, M., Agius, R., and Stout, R. (
Stone, P. H., and Godleski, J. J. (
Tao, F., González-Flecha, B., and Kobzik, L. (
Tarkiainen, T. H., Timonen, K. L., Vanninen, E. J., Alm, S., Hartikainen, J. E. K., and Pekkanen, J. (
Touyz, R. M., and Schiffrin, E. L. (
Van Eeden, S. F., and Hogg, J. C. (
Watkinson, W. P., Campen, M. J., and Costa, D. L. (
Author notes
*Department of Pathology, School of Medicine, University of São Paulo, São Paulo, Brazil; †Pulmonary Division, School of Medicine, University of São Paulo, Experimental Air Pollution Laboratory, São Paulo, Brazil; ‡Institute of Research in Nuclear Energy of the State of São Paulo, São Paulo, Brazil; §Department of Environmental Health, Physiology Program, Harvard School of Public Health, Boston, Massachusetts
- iron
- vascular constriction
- inflammation
- pulmonary vasculature
- lung
- antimony
- cobalt
- chlorine
- air pollution
- arteriole
- brazil
- bromine
- cardiovascular system
- lanthanum
- lymphocytes
- rats, wistar
- reticulocytes
- scandium
- sulfur
- thorium
- arsenic
- fibrinogen assay
- heart
- hematocrit
- manganese
- morbidity
- rats
- trachea
- segmented neutrophils
- fiberglass
- particulate matter
- filters
- histopathology tests




Comments