-
PDF
- Split View
-
Views
-
Cite
Cite
Leen J M Seys, W Widagdo, Fien M Verhamme, Alex Kleinjan, Wim Janssens, Guy F Joos, Ken R Bracke, Bart L Haagmans, Guy G Brusselle, DPP4, the Middle East Respiratory Syndrome Coronavirus Receptor, is Upregulated in Lungs of Smokers and Chronic Obstructive Pulmonary Disease Patients, Clinical Infectious Diseases, Volume 66, Issue 1, 1 January 2018, Pages 45–53, https://doi.org/10.1093/cid/cix741
Close - Share Icon Share
Abstract
Middle East respiratory syndrome coronavirus (MERS-CoV) causes pneumonia with a relatively high case fatality rate in humans. Smokers and chronic obstructive pulmonary disease (COPD) patients have been reported to be more susceptible to MERS-CoV infection. Here, we determined the expression of MERS-CoV receptor, dipeptidyl peptidase IV (DPP4), in lung tissues of smokers without airflow limitation and COPD patients in comparison to nonsmoking individuals (never-smokers).
DPP4 expression was measured in lung tissue of lung resection specimens of never-smokers, smokers without airflow limitation, COPD GOLD stage II patients and in lung explants of end-stage COPD patients. Both control subjects and COPD patients were well phenotyped and age-matched. The mRNA expression was determined using qRT-PCR and protein expression was quantified using immunohistochemistry.
In smokers and subjects with COPD, both DPP4 mRNA and protein expression were significantly higher compared to never-smokers. Additionally, we found that both DPP4 mRNA and protein expression were inversely correlated with lung function and diffusing capacity parameters.
We provide evidence that DPP4 is upregulated in the lungs of smokers and COPD patients, which could partially explain why these individuals are more susceptible to MERS-CoV infection. These data also highlight a possible role of DPP4 in COPD pathogenesis.
Middle East Respiratory Syndrome coronavirus (MERS-CoV) is a newly emerging pathogen that mainly causes pneumonia with a relatively high case-fatality rate [1, 2]. Since 2012, ~1900 laboratory-confirmed cases have been reported to the World Health Organization (WHO) [2]. The majority of cases occurred in familial or hospital-related clusters through human-to-human transmission [3–5]. The clinical course of MERS-CoV infection ranges from asymptomatic to acute respiratory distress syndrome with need for ventilatory support [3, 5–7]. To infect its host, MERS-CoV attaches to its receptor, an exopeptidase called dipeptidyl peptidase 4 (DPP4), also known as CD26 [8].
DPP4 is a type II transmembrane glycoprotein that is expressed in many cell types and organs in the body. It serves multiple functions among which post-translational cleavage of hormones and chemokines, T-cell activation, cell adhesion, and apoptosis [9–11]. In lungs, however, DPP4 is expressed at a minimum level [12], mainly in alveolar epithelial cells and endothelial cells, and to a lesser extent in bronchiolar epithelial cells, airway submucosal glands, alveolar macrophages, lymphocytes, and plasmacytoid dendritic cells [13–16]. Importantly, the alveolar epithelial cells are the main target for MERS-CoV [13, 17].
Several underlying comorbidities, including chronic lung diseases, have been reported to increase the risk of acquiring MERS-CoV infection [18]. Chronic obstructive pulmonary disease (COPD) is a highly prevalent chronic lung disease in older subjects and is currently the leading cause of death worldwide [19, 20]. The most common cause of COPD is chronic cigarette smoking. The inflammatory response to cigarette smoke results in an excessive release of chemokines and cytokines with a subsequent high influx of immune cells [20]. Because smoking has also been reported to increase susceptibility to MERS-CoV infection [18], we aimed to investigate the expression of the MERS-CoV receptor, DPP4, in a large well-phenotyped cohort of smokers, with and without airflow limitation, in comparison to age-matched individuals that never smoked (never-smokers).
METHODS
Human Lung Tissue Samples
Lung resection specimens were obtained from patients diagnosed with solitary pulmonary tumors at Ghent University Hospital (Ghent, Belgium) or from explant lungs from end-stage COPD patients (UZ Gasthuisberg, Leuven, Belgium). Based on preoperative spirometry, diffusion capacity tests and questionnaires, patients were categorized as never-smokers with normal lung function, smokers without airflow limitation or patients with COPD. COPD severity was defined according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) classification [19]. None of the patients were treated with neo-adjuvants chemotherapy. Lung tissue of patients diagnosed with solitary pulmonary tumor was obtained at a maximum distance from the pulmonary lesions and without signs of retro-obstructive pneumonia or tumor invasion and collected by a pathologist. Lung tissue of patients with COPD GOLD III-IV was obtained from lung explants of end-stage COPD patients undergoing lung transplantation. Written informed consent was obtained from all subjects. This study was approved by the medical ethical committees of the Ghent University Hospital (2011/114) and the University Hospital Gasthuisberg Leuven (S51577). Patient characteristics are listed in Table 1. Detailed patient characteristics per read-out are provided in supplementary Tables S1 and S2.
Characteristics of study population (n = 117)
| . | Never-smokers . | Smokersa . | COPD IIb . | COPD III–-IVc . |
|---|---|---|---|---|
| Number | 21 | 32 | 37 | 27 |
| Sex (M/F) | 6/15d | 23/9d | 34/3d | 12/14d |
| Age (years) | 65 (58–71) | 64.5 (55–71) | 65 (58–69) | 56.5 (54–60)e,f,g |
| Current- / ex-smoker | - | 19/13d | 24/13d | 0/27d |
| Smoking history (PY) | 0 (0–0) | 33 (14–51)e | 45 (40–60)e,f | 30 (25–36)e,g |
| FEV1 post (L) | 2,4 (2,1–3) | 2,7 (2,3–3,3) | 2,1 (1,8–2,4)e,f | 0,7 (0,5–1)e,f,g |
| FEV1 post (% predicted) | 103 (92–117) | 95 (92–112) | 69 (61–75)e,f | 27 (21–33)e,f,g |
| FEV1 / FVC post (%) | 78 (74–83) | 76 (72–79) | 56 (51–61)e,f | 30 (27–35)e,f,g |
| DLCO (% predicted) | 88 (81–103) | 83 (65–104) | 67 (51–86)e,f | 34 (32–37)e,f,g |
| KCO (% predicted) | 95 (86–121) | 93 (78–106) | 85 (65–107)e | 52 (46–59)e,f,g |
| ICS (yes/no) | 1/19d | 2/30d | 16/21d | 25/1d |
| . | Never-smokers . | Smokersa . | COPD IIb . | COPD III–-IVc . |
|---|---|---|---|---|
| Number | 21 | 32 | 37 | 27 |
| Sex (M/F) | 6/15d | 23/9d | 34/3d | 12/14d |
| Age (years) | 65 (58–71) | 64.5 (55–71) | 65 (58–69) | 56.5 (54–60)e,f,g |
| Current- / ex-smoker | - | 19/13d | 24/13d | 0/27d |
| Smoking history (PY) | 0 (0–0) | 33 (14–51)e | 45 (40–60)e,f | 30 (25–36)e,g |
| FEV1 post (L) | 2,4 (2,1–3) | 2,7 (2,3–3,3) | 2,1 (1,8–2,4)e,f | 0,7 (0,5–1)e,f,g |
| FEV1 post (% predicted) | 103 (92–117) | 95 (92–112) | 69 (61–75)e,f | 27 (21–33)e,f,g |
| FEV1 / FVC post (%) | 78 (74–83) | 76 (72–79) | 56 (51–61)e,f | 30 (27–35)e,f,g |
| DLCO (% predicted) | 88 (81–103) | 83 (65–104) | 67 (51–86)e,f | 34 (32–37)e,f,g |
| KCO (% predicted) | 95 (86–121) | 93 (78–106) | 85 (65–107)e | 52 (46–59)e,f,g |
| ICS (yes/no) | 1/19d | 2/30d | 16/21d | 25/1d |
Abbreviations: COPD, chronic obstructive pulmonary disease; DLCO, diffusing capacity of the lung for carbon monoxide; FEV1 forced expiratory volume in 1 second; FVC, forced vital capacity; IQR, interquartile range; KCO, transfer of carbon monoxide coefficient, ICS, inhaled corticosteroids; PY, pack years.
aSmokers without airflow limitation,
bsubjects with COPD stage II as defined by the Global initiative for Obstructive Lung Disease (GOLD).
Data are presented as median (IQR), Mann-Whitney U test:cSubjects with COPD stage III–IV as defined by GOLD.
Fisher’s exact test:dP < .001.
eP < .05 versus never smokers;
fP < .05 versus smokers without COPD;
gP < .05 versus COPD GOLD I–-II.
Characteristics of study population (n = 117)
| . | Never-smokers . | Smokersa . | COPD IIb . | COPD III–-IVc . |
|---|---|---|---|---|
| Number | 21 | 32 | 37 | 27 |
| Sex (M/F) | 6/15d | 23/9d | 34/3d | 12/14d |
| Age (years) | 65 (58–71) | 64.5 (55–71) | 65 (58–69) | 56.5 (54–60)e,f,g |
| Current- / ex-smoker | - | 19/13d | 24/13d | 0/27d |
| Smoking history (PY) | 0 (0–0) | 33 (14–51)e | 45 (40–60)e,f | 30 (25–36)e,g |
| FEV1 post (L) | 2,4 (2,1–3) | 2,7 (2,3–3,3) | 2,1 (1,8–2,4)e,f | 0,7 (0,5–1)e,f,g |
| FEV1 post (% predicted) | 103 (92–117) | 95 (92–112) | 69 (61–75)e,f | 27 (21–33)e,f,g |
| FEV1 / FVC post (%) | 78 (74–83) | 76 (72–79) | 56 (51–61)e,f | 30 (27–35)e,f,g |
| DLCO (% predicted) | 88 (81–103) | 83 (65–104) | 67 (51–86)e,f | 34 (32–37)e,f,g |
| KCO (% predicted) | 95 (86–121) | 93 (78–106) | 85 (65–107)e | 52 (46–59)e,f,g |
| ICS (yes/no) | 1/19d | 2/30d | 16/21d | 25/1d |
| . | Never-smokers . | Smokersa . | COPD IIb . | COPD III–-IVc . |
|---|---|---|---|---|
| Number | 21 | 32 | 37 | 27 |
| Sex (M/F) | 6/15d | 23/9d | 34/3d | 12/14d |
| Age (years) | 65 (58–71) | 64.5 (55–71) | 65 (58–69) | 56.5 (54–60)e,f,g |
| Current- / ex-smoker | - | 19/13d | 24/13d | 0/27d |
| Smoking history (PY) | 0 (0–0) | 33 (14–51)e | 45 (40–60)e,f | 30 (25–36)e,g |
| FEV1 post (L) | 2,4 (2,1–3) | 2,7 (2,3–3,3) | 2,1 (1,8–2,4)e,f | 0,7 (0,5–1)e,f,g |
| FEV1 post (% predicted) | 103 (92–117) | 95 (92–112) | 69 (61–75)e,f | 27 (21–33)e,f,g |
| FEV1 / FVC post (%) | 78 (74–83) | 76 (72–79) | 56 (51–61)e,f | 30 (27–35)e,f,g |
| DLCO (% predicted) | 88 (81–103) | 83 (65–104) | 67 (51–86)e,f | 34 (32–37)e,f,g |
| KCO (% predicted) | 95 (86–121) | 93 (78–106) | 85 (65–107)e | 52 (46–59)e,f,g |
| ICS (yes/no) | 1/19d | 2/30d | 16/21d | 25/1d |
Abbreviations: COPD, chronic obstructive pulmonary disease; DLCO, diffusing capacity of the lung for carbon monoxide; FEV1 forced expiratory volume in 1 second; FVC, forced vital capacity; IQR, interquartile range; KCO, transfer of carbon monoxide coefficient, ICS, inhaled corticosteroids; PY, pack years.
aSmokers without airflow limitation,
bsubjects with COPD stage II as defined by the Global initiative for Obstructive Lung Disease (GOLD).
Data are presented as median (IQR), Mann-Whitney U test:cSubjects with COPD stage III–IV as defined by GOLD.
Fisher’s exact test:dP < .001.
eP < .05 versus never smokers;
fP < .05 versus smokers without COPD;
gP < .05 versus COPD GOLD I–-II.
Human Proximal Bronchi Samples
Biopsy samples of proximal bronchi were obtained from 21 patients (17 male and 4 female) with moderate-to-severe COPD previously recruited for a separate study [21]. Inclusion criteria were the following: chronic productive cough, age between 40 and 70 years, current smokers, negative skin tests for inhalation allergens, FEV1 < 70% of predicted normal value or FEV1/VC < 0.70, reversibility of FEV1 < 10% pred after 750 µg terbutaline inhalation, and suffering from moderate-to-severe bronchial hyper-responsiveness, as determined by PC20 value upon challenge with histamine and methacholine. Exclusion criteria were a history of asthma, complaints of wheezing, recent respiratory tract infection, and recent or concurrent usage of anti-inflammatory drugs. Oral anti-inflammatory medication was discontinued for at least 3 months and inhaled glucocorticoids at least 6 weeks before the start of the study. Bronchoscopy was performed with an Olympus BF 1T10. At least 6 biopsies were taken from the bronchi of the right and the left upper and lower lobes using a fenestrated forceps (FB-18C or FB-20C). All was according to published guidelines [22]. The study was approved by the Medical Ethics Committee of the Erasmus University Medical Center Rotterdam, and written informed consent was obtained from all participants. Patient characteristics are listed in Table 2. Proximal bronchi biopsy samples of 16 healthy individuals (8 male and 8 female), previously described in the earlier study [23], were used as negative control.
Characteristics of the Patients in Which Proximal Bronchi Biopsy Samples Were Obtained
| . | Mean ± SD . | Median . | Range . |
|---|---|---|---|
| Age, years | 56.3 ± 8.9 | 60 | 42–46 |
| Actual smoking, cigarettes/day | 15.4 ± 7.4 | 13 | 6–30 |
| Pack-years | 25.3 ± 11.2 | 21 | 5–50 |
| FEV1, % predicted | 62.5 ± 12.9 | 65 | 34–93 |
| Reversibility, % predicted | 5.3 ± 3.1 | 5 | 0–9.0 |
| PC20, mg/ml | |||
| For histamine | 1.7 ± 2.1 | 0.87 | 0.11–8 |
| For methacholine | 4.6 ± 5.5 | 1.72 | 0.6–17.4 |
| . | Mean ± SD . | Median . | Range . |
|---|---|---|---|
| Age, years | 56.3 ± 8.9 | 60 | 42–46 |
| Actual smoking, cigarettes/day | 15.4 ± 7.4 | 13 | 6–30 |
| Pack-years | 25.3 ± 11.2 | 21 | 5–50 |
| FEV1, % predicted | 62.5 ± 12.9 | 65 | 34–93 |
| Reversibility, % predicted | 5.3 ± 3.1 | 5 | 0–9.0 |
| PC20, mg/ml | |||
| For histamine | 1.7 ± 2.1 | 0.87 | 0.11–8 |
| For methacholine | 4.6 ± 5.5 | 1.72 | 0.6–17.4 |
Abbreviations: FEV1, forced expiratory volume in 1 second; SD, standard deviation; PC20, provocative concentration causing a 20% fall in FEV1.
Characteristics of the Patients in Which Proximal Bronchi Biopsy Samples Were Obtained
| . | Mean ± SD . | Median . | Range . |
|---|---|---|---|
| Age, years | 56.3 ± 8.9 | 60 | 42–46 |
| Actual smoking, cigarettes/day | 15.4 ± 7.4 | 13 | 6–30 |
| Pack-years | 25.3 ± 11.2 | 21 | 5–50 |
| FEV1, % predicted | 62.5 ± 12.9 | 65 | 34–93 |
| Reversibility, % predicted | 5.3 ± 3.1 | 5 | 0–9.0 |
| PC20, mg/ml | |||
| For histamine | 1.7 ± 2.1 | 0.87 | 0.11–8 |
| For methacholine | 4.6 ± 5.5 | 1.72 | 0.6–17.4 |
| . | Mean ± SD . | Median . | Range . |
|---|---|---|---|
| Age, years | 56.3 ± 8.9 | 60 | 42–46 |
| Actual smoking, cigarettes/day | 15.4 ± 7.4 | 13 | 6–30 |
| Pack-years | 25.3 ± 11.2 | 21 | 5–50 |
| FEV1, % predicted | 62.5 ± 12.9 | 65 | 34–93 |
| Reversibility, % predicted | 5.3 ± 3.1 | 5 | 0–9.0 |
| PC20, mg/ml | |||
| For histamine | 1.7 ± 2.1 | 0.87 | 0.11–8 |
| For methacholine | 4.6 ± 5.5 | 1.72 | 0.6–17.4 |
Abbreviations: FEV1, forced expiratory volume in 1 second; SD, standard deviation; PC20, provocative concentration causing a 20% fall in FEV1.
Table 3 provides an overview of the different cohorts and samples used in this study.
Overview of the Cohorts and Samples Used in This Study
| Overview of cohorts and samples used |
| 90 patients (21 never-smokers, 32 smokers without airflow limitation and 37 patients with COPD GOLD stage II) who underwent lobectomia or pneumectomia due to lung cancer. |
| •73/90 patients: samples for both qRT-PCR and IHC analyses. •5/90 patients: samples only for qRT-PCR analysis. •12/90 patients: samples only for IHC analysis. |
| 27 patients with COPD GOLD stage III–IV who underwent lung transplantation due to end-stage COPD. |
| •14/27 patients: samples for qRT-PCR analysis. •13/27 patients: samples for IHC analysis. |
| 37 patients who underwent bronchial biopsies. |
| •21/37 patients with moderate-to-severe COPD (ref): samples used for IHC staining. •16/37 control patients with airflow limitation (ref): samples used for IHC staining. |
| Overview of cohorts and samples used |
| 90 patients (21 never-smokers, 32 smokers without airflow limitation and 37 patients with COPD GOLD stage II) who underwent lobectomia or pneumectomia due to lung cancer. |
| •73/90 patients: samples for both qRT-PCR and IHC analyses. •5/90 patients: samples only for qRT-PCR analysis. •12/90 patients: samples only for IHC analysis. |
| 27 patients with COPD GOLD stage III–IV who underwent lung transplantation due to end-stage COPD. |
| •14/27 patients: samples for qRT-PCR analysis. •13/27 patients: samples for IHC analysis. |
| 37 patients who underwent bronchial biopsies. |
| •21/37 patients with moderate-to-severe COPD (ref): samples used for IHC staining. •16/37 control patients with airflow limitation (ref): samples used for IHC staining. |
In this study we used resection lung tissue of 90 patients who underwent lobectomia/pneumectomia due to lung cancer, explant lung tissue of 27 end-stage COPD patients and bronchial biopsy tissue of 37 patients.
Abbreviations: COPD, chronic obstructive pulmonary disease; GOLD, global initiative for chronic obstructive lung disease; IHC, immunohistochemistry; qRT-PCR, quantitative reverse-transcription polymerase chain reaction.
Overview of the Cohorts and Samples Used in This Study
| Overview of cohorts and samples used |
| 90 patients (21 never-smokers, 32 smokers without airflow limitation and 37 patients with COPD GOLD stage II) who underwent lobectomia or pneumectomia due to lung cancer. |
| •73/90 patients: samples for both qRT-PCR and IHC analyses. •5/90 patients: samples only for qRT-PCR analysis. •12/90 patients: samples only for IHC analysis. |
| 27 patients with COPD GOLD stage III–IV who underwent lung transplantation due to end-stage COPD. |
| •14/27 patients: samples for qRT-PCR analysis. •13/27 patients: samples for IHC analysis. |
| 37 patients who underwent bronchial biopsies. |
| •21/37 patients with moderate-to-severe COPD (ref): samples used for IHC staining. •16/37 control patients with airflow limitation (ref): samples used for IHC staining. |
| Overview of cohorts and samples used |
| 90 patients (21 never-smokers, 32 smokers without airflow limitation and 37 patients with COPD GOLD stage II) who underwent lobectomia or pneumectomia due to lung cancer. |
| •73/90 patients: samples for both qRT-PCR and IHC analyses. •5/90 patients: samples only for qRT-PCR analysis. •12/90 patients: samples only for IHC analysis. |
| 27 patients with COPD GOLD stage III–IV who underwent lung transplantation due to end-stage COPD. |
| •14/27 patients: samples for qRT-PCR analysis. •13/27 patients: samples for IHC analysis. |
| 37 patients who underwent bronchial biopsies. |
| •21/37 patients with moderate-to-severe COPD (ref): samples used for IHC staining. •16/37 control patients with airflow limitation (ref): samples used for IHC staining. |
In this study we used resection lung tissue of 90 patients who underwent lobectomia/pneumectomia due to lung cancer, explant lung tissue of 27 end-stage COPD patients and bronchial biopsy tissue of 37 patients.
Abbreviations: COPD, chronic obstructive pulmonary disease; GOLD, global initiative for chronic obstructive lung disease; IHC, immunohistochemistry; qRT-PCR, quantitative reverse-transcription polymerase chain reaction.
Purification of Human Lung Dendritic Cell-subsets
Lung dendritic cells (DC) were isolated from single cell suspensions of lung tissue of 3 patients, as described previously [24]. Lung tissues were rinsed, cut into small fragments, and incubated in digestion medium. Next, the samples were resuspended in Ca2+ and Mg2+–free PBS containing 10 mM EDTA and passed through a 40 µm filter. Subsequently, pulmonary mononuclear cells were separated on a Ficoll density gradient. The cells were labeled with anti-CD3-FITC, anti-CD19-FITC, anti-CD207-PE, anti-CD209-PerCp-Cy5 and anti-BDCA2-APC and sorted on a FACSAria (BD Biosciences).
RNA Extraction and Real-Time Polymerase Chain Reaction Analysis
RNA extraction and polymerase chain reaction (PCR) analysis of lung tissue were performed as described previously [25]. RNA extraction from lung tissue blocks of 92 subjects (18 never-smokers, 26 smokers without airflow limitation, 34 patients with COPD GOLD II, 14 patients with COPD GOLD IV) was performed with the miRNeasy Mini kit (Qiagen, Hilden, Germany), following manufacturer’s instructions. Next, complementary DNA (cDNA) was prepared with the iScript™ Advanced cDNA Synthesis Kit for RT-qPCR (Bio-Rad, Hercules, California). Taqman Gene Expression Assays (Applied Biosystems, Forster City, California) were used to measure the expression of DPP4 and the reference genes Glyceraldehyde-3-phosphate dehydrogenase (GAPDH), Hypoxanthine phosphoribosyltransferase-1 (HPRT-1) and Succinate Dehydrogenase complex flavoprotein subunit A (SDHA). Data were analyzed using the standard curve method, and expression of DPP4 was calculated relative to the expression of the 3 reference genes, using the geNorm applet according to the guidelines and theoretical framework previously described [25, 26].
For human lung DC subsets, RNA extraction was performed with miRNeasy Mini kit (Qiagen, Hilden, Germany), whereas RNA amplification was with the Qiagen QuantiTect Whole Transcriptome kit, both following manufacturer’s instructions. DPP4 expression in the DC subsets was calculated relative to the expression of GAPDH, HPRT1 and peptidylprolyl isomerase A (PPIA) as described previously [25].
DPP4 Immunohistochemistry and Analyses
Sections obtained from formalin-fixed paraffin-embedded lung tissue blocks of 98 subjects (19 never-smokers, 30 smokers without COPD, 36 subjects with COPD GOLD II, and 13 subjects with COPD GOLD III-IV) were incubated with anti-DPP4 antibody (polyclonal goat-anti-human, R&D Systems, AF1180) [15] after antigen retrieval with citrate buffer (Klinipath, Olen, Belgium). Next, slides were colored with diaminobenzidine (Dako, Carpinteria, California) and counterstained with Mayer’s hematoxylin (Sigma-Aldrich, St-Louis, Missouri). The isotype control was goat immunoglobulin G (IgG) from R&D Systems (Abingdon, UK) (AB-108-C). To co-stain DPP4 with alveolar epithelial cells, anti-aquaporin 5 (Abcam, Cambridge, UK) (ab92320) and pro-surfactant C (Abcam) (ab90716) were used to detect, respectively, type I and type II alveolar cells and subsequently colored with Vector Blue (Vector, Peterborough, UK).
Quantitative scoring of the amount of DPP4-positive scoring in alveolar tissue and airway epithelium was performed using the Axiovision software (Zeiss, Oberkochen, Germany). In order to measure the area of DPP4-positive signal in alveolar tissue, 15 images of alveolar tissue were recorded from an average of 3 tissue blocks per patient. The intensity of brown staining we wished to score was selected by means of selecting specific hue, lightness, and saturation values. The hue, saturation, and lightness values were identical for all images, therefore restricting our scoring to a specific signal. In every image the alveolar tissue was selected and the DPP4-positive signal was calculated only within the alveolar tissue and normalized to the area of alveolar tissue present in each image. The final score of each patient was the average ratio of DPP4-positive signal of the 15 images. In the airway epithelium, the amount of DPP4 signal was normalized to the length of the basement membrane (Pbm). The final score of each patient was the average DPP4 staining in all airways present in all tissue blocks available of that patient. The number of airways per patient was between 3 and 20.
DPP4 detection in the frozen samples of proximal bronchi was performed with 1 µg/mL mouse anti-DPP4 monoclonal antibody (Santa Cruz Biotechnology, Dallas, Texas) [15], after previously fixed with acetone and incubated with 10% normal goat serum (Dako, Glostrup, Denmark) for 1 hour at room temperature. These slides were subsequently stained with biotinylated goat antimouse Ig serum (1:50 in PBS/BSA plus 10% human serum) and with streptavidin alkaline phosphatase (1:50 in PBS/BSA plus 10% human serum; Biogenex, Klinipath, Duiven, The Netherlands) for 30 minutes each. A positive signal was revealed with New Fuchsin substrate (Chroma, Kongen, Germany). Counterstaining was performed with Gill’s hematoxylin. Negative control staining was performed by the substitution of the primary monoclonal antibody with an isotype antibody.
Statistical Analysis
Statistical analysis was performed with Sigma Stat software (SPSS 23.0, Chicago, Illinois), using Kruskal-Wallis, Mann-Whitney U, Fisher exact test, and Spearman correlation analysis. In addition, one-way analysis of variance (ANOVA) and T-tests were used for statistical analyses of the DC subsets. Characteristics of the study population are presented as a median and interquartile range. Differences at P-values < .05 were considered to be significant (*P < .05, **P < .01, and *** P < .001).
RESULTS
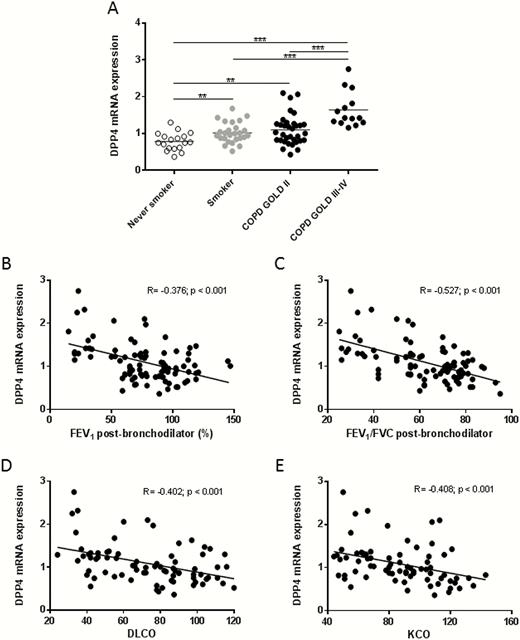
DPP4 mRNA Expression is Upregulated in Lungs of Smokers and COPD Patients
Messenger RNA (mRNA) expression of DPP4 was analyzed in lung tissue of 92 subjects. Lung tissue was derived from either resection tissue of lobectomy (never smokers, smokers without airflow limitation and patients with COPD GOLD stage II) or explant lungs of lung transplantation (patients with COPD GOLD stage III–IV). Patient characteristics are described in supplementary Table 1.
Compared to never-smokers, mRNA expression of DPP4 in lung tissue of smokers without airflow limitation and patients with COPD was significantly increased (Figure 1A). Moreover, DPP4 mRNA expression in lung tissue of patients with COPD GOLD stage III–IV was significantly higher than in lung tissue of smokers without airflow limitation and patients with COPD GOLD stage II (Figure 1A). Quantification according to smoking status (ex- vs. current smokers) is shown in Supplementary Figure S1. Furthermore, DPP4 mRNA expression was inversely correlated with the severity of airflow limitation: FEV1 (R = −0.376, P < .001) and FEV1/FVC ratio (R = −0.527, P < .001) (Figure 1B–C). In addition, the mRNA expression of DPP4 was also correlated inversely with the diffusing capacity of the lung, DLCO (R = −0.402, P < .001) and KCO (R = −0.408, P < .001) (Figure 1D–E). Linear regression analysis revealed that the association of DPP4 mRNA expression with the presence of COPD was significant even when corrected for age, sex, pack-years, and use of inhaled corticosteroids (Supplementary Table S3).
DPP4 mRNA expression in the lung tissues of smokers and COPD patients. A, DPP4 mRNA expression was measured by qRT-PCR and normalized to three reference genes (GAPDH, HPRT-1, SDHA). DPP4 mRNA expression in the lungs of smokers and COPD patients is significantly higher in comparison to that of never smokers. B, Correlation of DPP4 mRNA expression with post-bronchodilator FEV1 values. C, Correlation of DPP4 mRNA expression with post-bronchodilator Tiffeneau index (FEV1/FVC). D, Correlation of DPP4 mRNA expression with DLCO (diffusing capacity or transfer factor of the lung for carbon monoxide). E, Correlation of DPP4 mRNA expression with KCO (carbon monoxide transfer coefficient). **P < .01, ***P < .001. Abbreviations: COPD, chronic obstructive pulmonary disease; DLCO, diffusing capacity of the lung for carbon monoxide; FEV/FVC, forced expiratory volume in 1 second/forced vital capacity; GOLD, global initiative for obstructive lung disease; KCO, transfer of carbon monoxide coefficient; mRNA, messenger RNA; qRT-PCR, quantitative reverse-transcription polymerase chain reaction.
Additionally, because dendritic cells (DCs) play a crucial role in antiviral immunity, we investigated whether DPP4 mRNA expression differs between DC subsets. Three DC subsets were sorted: langerin-positive DCs, DC-SIGN-positive DCs, and plasmacytoid DCs (pDCs). DPP4 mRNA was merely detected in pDCs (Supplementary Figure S2).
DPP4 Protein Expression is Upregulated in Lungs of Smokers and COPD Patients
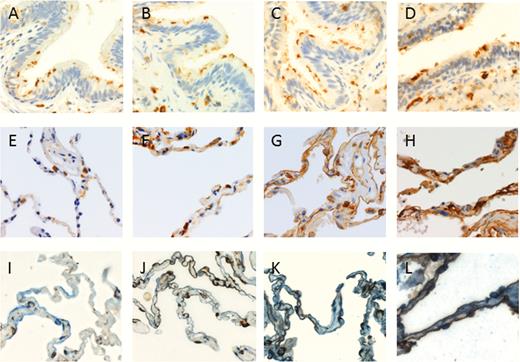
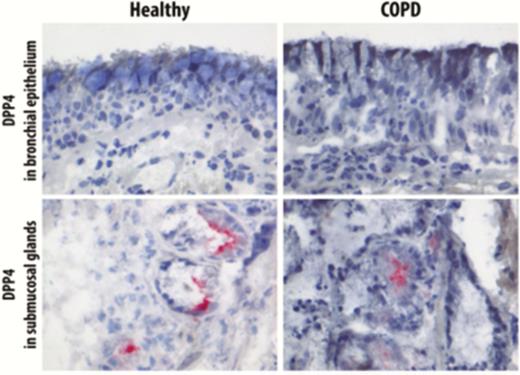
DPP4 protein expression was studied in lung tissue of never-smokers, smokers without airflow limitation, and COPD patients by using immunohistochemistry (IHC) staining. DPP4 was detected on the apical surface of bronchiolar epithelium and in the alveolar epithelial cells. In the alveoli, we observed that DPP4 protein was gradually increased from never-smokers to COPD GOLD stage III–IV (Figure 2). Additionally, we performed immunohistochemical staining of DPP4 with both aquaporin 5 (marker of type I alveolar epithelial cells) and pro-surfactant C (marker of type II alveolar epithelial cells), confirming that the upregulation of DPP4 protein can mainly be contributed to the alveolar epithelial cells (Figure 2I–L). In contrast, this increment was not observed in the bronchiolar epithelium (Figure 2A), as well as in the proximal bronchial epithelium (Figure 3). Furthermore, DPP4 was also detected in the endothelial cells, alveolar macrophages, immune cells in the submucosal region of airway epithelium, and lymphoid aggregates (Supplementary Figure S3). We further quantified DPP4 signals in the lung tissues of 98 subjects using the Axiovision software (Zeiss). Characteristics of these patients are presented in supplementary Table 2.
DPP4 protein expression in the bronchiolar epithelium and the alveolar tissues of never smoker, smoker, and COPD patients. Representative images of DPP4 staining in the bronchiolar epithelium (top row) and alveoli (middle and bottom row) of A,E,I, never-smoker, B,F,J, smoker without airflow limitation, C,G,K, subject with COPD GOLD stage II and D,H,L, subject with COPD GOLD stage III–IV. I–-L, are immunohistochemical stainings of DPP4 (brown) and aquaporin 5 (marker of type I alveolar epithelial cells) and pro-surfactant C (marker of type II alveolar epithelial cells) (both in blue). Co-staining of DPP4 with either one of the alveolar epithelial cell types results in a dark brown stain. DPP4 was mainly expressed in the alveolar epithelial cells and expressed the most intense in the COPD GOLD stage III–IV group. A 400× magnification was used for all photomicrographs in this figure. Abbreviation: COPD, chronic obstructive pulmonary disease; GOLD, global initiative for chronic obstructive lung disease.
DPP4 staining in the proximal bronchi epithelium. Representative images of DPP4 staining in proximal bronchial epithelium and submucosal glands of the healthy control subject with COPD GOLD stage II. DPP4 was hardly detected in the apical surface of the proximal bronchi epithelium of both healthy control and COPD patients. Submucosal glands here served as positive control for DPP4 staining. Abbreviation: COPD, chronic obstructive pulmonary disease.
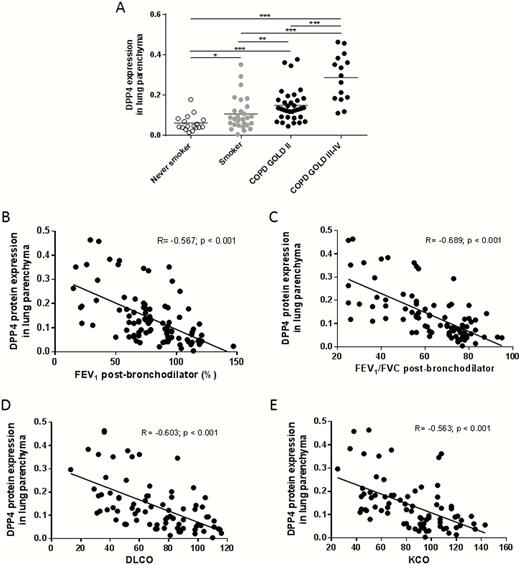
Compared to never-smokers, DPP4 protein expression was significantly increased in the alveolar epithelial cells of smokers and patients with COPD. DPP4 protein expression was the highest in patients with COPD GOLD stage III–IV (Figure 4A). Quantification of DPP4 protein expression according to smoking status (ex- vs. current smoking) is shown in Supplementary Figure S4. Similar to DPP4 mRNA expression, DPP4 protein was also inversely correlated with lung function parameters FEV1 (R = −0.567, P < .001) and FEV1/FVC ratio (R = −0.689, P < .001); as well as diffusing capacity parameters DLCO (R = −0.603, P < .001) and KCO (R = −0.563, P < .001). Linear regression analysis revealed that the association of alveolar DPP4 expression with the presence of COPD was significant even when corrected for age, gender, pack-years, and use of inhaled corticosteroids (Supplementary Table S4).
DPP4 protein expression in the lung tissues of smokers and COPD patients. , DPP4 protein expression was analyzed by using Axiovision software (Zeiss). The area of DPP4 positive signal was normalized to the total area of cells present in each analyzed image. DPP4 protein expression in the lungs of smokers and COPD patients is significantly higher in comparison to that of never smokers. B, Correlation of alveolar DPP4 protein expression with post-bronchodilator FEV1 values. C, Correlation of alveolar DPP4 protein expression with post-bronchodilator Tiffeneau index (FEV1/FVC). D, Correlation of alveolar DPP4 protein expression with DLCO (diffusing capacity or transfer factor of the lung for carbon monoxide). E, Correlation of alveolar DPP4 protein expression with KCO (carbon monoxide transfer coefficient). **P < .01, ***P < .001. Abbreviation: COPD, chronic obstructive pulmonary disease.
DISCUSSION
Our study investigated the expression of the MERS-CoV receptor, DPP4, in lung tissues of smokers without airflow limitation and COPD patients in comparison to never-smokers. As previously reported, DPP4 is mainly detected in the alveolar epithelial cells of the lungs, the main target of MERS-CoV infection [13, 15]. Among the dendritic cells, we found that DPP4 mRNA is mainly expressed in pDCs; confirming in vitro data showing that among the antigen presenting cells, pDCs produce large amounts of type I and III interferon upon contact with MERS-CoV [14]. Most importantly, we provide evidence that DPP4 is upregulated in the lungs, both at mRNA and protein level, not only in COPD patients but also in that of smokers. This indicates that these individuals may be more susceptible to MERS-CoV, supporting both smoking and COPD as risk factors for MERS-CoV infection [18]. These results are in line with a recent study describing a higher DPP4 expression in lungs of 4 COPD patients compared to 16 control subjects of different ages [13].
In this study, we did not find any evidence of DPP4 upregulation in the bronchial and bronchiolar epithelium in the lungs of smokers and COPD patients, suggesting that DPP4 upregulation in pulmonary epithelia is restricted to the alveolar epithelial cells. Previous studies have shown that DPP4 is limitedly expressed in the bronchial and bronchiolar epithelium, and even absent at the apical surface of the nasal respiratory and olfactory epithelium of humans [13, 15]. Future studies are needed to assess whether DPP4 upregulation is specific for the alveolar epithelial cells or also occurs in the upper respiratory tract epithelium. Additionally, the importance of alveolar macrophages in the pathogenesis of MERS-CoV needs further research as these cells also express DPP4 and patrol the alveoli while being in close contact with the alveolar epithelial cells.
It is currently unclear how DPP4 is upregulated in the lungs of smokers and COPD patients. Several cytokines have been reported to upregulate DPP4 in vitro. TGF-β2, for instance, could upregulate DPP4 protein expression and enzymatic activity in primary human endothelial cells [27], whereas interleukin (IL) 13 has been reported to increase DPP4 mRNA expression in human primary bronchial epithelial cells [28]. On the other hand, in COPD pathogenesis, several cytokines—such as IL-6, IL-8, and the TGF-β superfamily—have been described to play important roles [29, 30]. Further studies are needed to identify cytokines that could both upregulate DPP4 in the lung and influence COPD pathogenesis.
We also showed that DPP4 mRNA and protein expression were inversely correlated with lung function and diffusing capacity parameters. These data suggest a possible role of DPP4 in COPD pathogenesis. DPP4 is an exopeptidase responsible for cleaving chemokines and this alters the biological function. Moreover, DPP4 is able to activate T cells and induce production of pro-inflammatory cytokines, which later could affect the development of COPD [20, 31–33]. Furthermore, DPP4 is also capable of influencing migration of immune cells by activating or deactivating chemokines in an inflammatory or tumor environment [10, 11]. Interestingly, soluble DPP4 in the serum of COPD patients has been reported to be significantly lower compared to that of non-COPD controls [34, 35]. It remains possible that in COPD patients, DPP4 concentration is low in the serum and high in the lungs to facilitate migration of certain immune cells into or out of the lungs.
Our study has several strengths; first, we included a large number of patients which have been thoroughly characterized. Second, to eliminate the possible interference of the presence of malignancy in our patients, we also included lung tissue derived from explant lungs of end-stage COPD patients, devoid of malignancy. A possible limitation of our study might be the sex imbalance in the groups with, respectively, a male predominance in the COPD groups and a female predominance in the never-smokers. However, it should be noted that linear regression analyses indicated that sex does not significantly contribute to the differences in expression of membrane-bound DPP4. Our data are in line with recent analyses of soluble DPP4 in serum in patients with COPD versus non-COPD controls indicating that there is no relationship between sex and DPP4 levels [35]. It is also important to acknowledge that there are other factors related to COPD that could contribute to the increased MERS-CoV susceptibility independent of DPP4. For instance, COPD patients are mostly in advanced age and more prone to many other pulmonary infections during hospitalization [36]. Besides that, COPD is associated with systemic inflammation, which might also cause insufficient host immune response against pathogens [20, 37].
In conclusion, because smoking is the most common etiology of COPD [19], our data highlight the association between chronic exposure to cigarette smoking and DPP4 upregulation in the lungs, as well as partially explain the increased susceptibility of smokers and COPD patients to MERS-CoV infection [18]. It is imperative to try replicating this observation in an animal model in order to further dissect the molecular pathway of DPP4 upregulation in the lungs.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgements. The authors thank Greet Barbier, Indra De Borle, Katleen De Saedeleer, Anouck Goethals, Marie-Rose Mouton, and Ann Neesen, from the laboratory for Translational Research in Obstructive Pulmonary Diseases, Department of Respiratory Medicine (Ghent University Hospital, Ghent, Belgium) for their excellent technical assistance. Furthermore, we thank Dr. Geert Van Pottelberge (Maatschap Longartsen Zeeuws-Vlaanderen / Dept. of Respiratory Medicine, ZorgSaam Ziekenhuis Zeeuws-Vlaanderen, Terneuzen, The Netherlands) for his contribution to the sorting of human DC and Prof. Bart Vanaudenaerde and Dr. Stijn Verleden (Department of Pneumology, Leuven) for providing us with the explant lungs of patients with severe chronic obstructive pulmonary disease.
Financial support. This work was supported by the Concerted Research Action of the Ghent University [grant BOF/GOA 01G02714]; the Fund for Scientific Research in Flanders (FWO Vlaanderen) and the Interuniversity Attraction Poles program [grant IUAP P7/30]; and the TOP Project grant from the Netherlands Organization for Health Research and Development (ZonMW) [grantr 91213066].
Potential conflicts of interest. W. J. reports grants from Boehringer Ingelheim, Astra Zeneca, Novartis, Chiesi, GSK, outside the submitted work; G. F. J. reports grants from AstraZeneca, grants from Boehringer Ingelheim, grants from Chiesi, grants from GlaxoSmithKline, grants and personal fees from Novartis, personal fees from Teva, outside the submitted work. All other authors have no reported disclosures. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
B. L. H. and G. G. B. contributed equally to this manuscript.





Comments