-
PDF
- Split View
-
Views
-
Cite
Cite
Thomas M. File, Clinical Efficacy of Newer Agents in Short-Duration Therapy for Community-Acquired Pneumonia, Clinical Infectious Diseases, Volume 39, Issue Supplement_3, September 2004, Pages S159–S164, https://doi.org/10.1086/421354
Close - Share Icon Share
Abstract
Streptococcus pneumoniae, the most important respiratory tract pathogen implicated in community-acquired pneumonia (CAP), is becoming increasingly resistant in vitro to the β-lactams and macrolides, and fluoroquinolone resistance has been detected. A growing body of evidence suggests that prolonged antimicrobial use may contribute directly and indirectly to increased antimicrobial resistance among common respiratory pathogens. Long-term exposure to antimicrobial agents, especially less-potent agents, directly increases selection pressure for resistance. Indirectly, poor patient compliance, multiple daily dosing, and the increased risk of adverse events further complicate the resistance issue and diminish the efficacy of long-term antimicrobial use. Controlled clinical trials addressing the appropriate duration of therapy for CAP are lacking. However, available data suggest that with appropriate antibiotic selection, based on appropriate spectrum, potency, and pharmacokinetic/pharmacodynamic profile, lower respiratory tract infections in outpatients can be successfully treated in <7 days rather than the 7–14 days currently recommended.
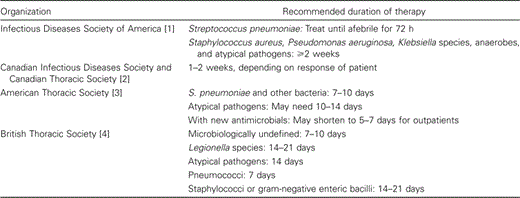
There is a dearth of controlled clinical trials addressing the appropriate duration of therapy for community-acquired pneumonia (CAP) [1–4]. Consequently, current recommendations regarding the duration of therapy for pneumonia are based primarily on traditional perceptions and differ from one professional organization to another (table 1). For example, the Infectious Diseases Society of America (IDSA) recommends that treatment of pneumococcal pneumonia be continued for 72 h after the patient becomes afebrile, whereas the American Thoracic Society generally suggests a 7- to 10-day course of therapy [1, 3]. For other pathogens, longer durations of therapy of 14–21 days are recommended. However, the American Thoracic Society most recently recommends that, with new agents that have a long serum or tissue half-life, it may be possible to shorten the duration of outpatient therapy to 5–7 days [3].
Guidelines for the treatment of community-acquired pneumonia: duration of therapy.
The goals of therapy are to eradicate the causative pathogen, promote resolution of clinical symptoms, and prevent emergence of resistant organisms [5]. There are potential advantages to short-course therapy in general and for CAP in particular. By reducing overall exposure to an antibiotic, a shorter course of therapy may reduce the selection pressure for the pathogen being treated and may decrease the impact on endogenous flora as well. Shorter-course therapy can potentially reduce the risk of adverse events that may occur with more prolonged antibiotic exposure. Medication regimens with once-daily dosing and abbreviated treatment duration are likely to enhance compliance. Improved compliance may also translate into cost savings. In brief, the concept for short-course therapy is to “hit hard and stop early.”
The ultimate goals of short-course therapy are early eradication of pathogens and reduction of selection pressure, with concomitant decreased propensity for development of resistance. However, to do this effectively, a shorter course of therapy must be based on sound pharmacokinetic and pharmacodynamic data. Specifically, an antimicrobial must be able to achieve adequate tissue penetration and drug concentration at the site of infection for a sufficient length of time [6, 7]. It is anticipated that, with appropriate antimicrobial agents, high clinical and microbiological cure rates will be possible with short-course regimens. Antimicrobial agents exhibit pharmacodynamic properties characterized by concentration-dependent killing with prolonged postantibiotic effects, measured by maximum concentration/MIC; concentration- and time-dependent killing with moderate to prolonged postantibiotic effects, measured by area under curve (AUC0–24)/MIC; and time-dependent killing (no postantibiotic effects) measured by time the antibiotic concentration exceeds the MIC. Providers can use their knowledge of each antibiotic's known pharmacodynamic profile to select the most appropriate agent, dosing amount, interval, and duration [8, 9].
Reduction of Selection Pressure For Resistance
A number of studies, 2 of which are reviewed here, illustrate the importance of short-course therapy in terms of resistance. An observational study of 941 French children aged 3–6 years found that a low daily dose and long duration (>5 days) of oral β-lactam therapy were independent predictors of pharyngeal carriage of penicillin-resistant Streptococcus pneumoniae [10]. Compared with no antibiotic treatment, use of a β-lactam antibiotic increased the risk of carriage of penicillin-resistant S. pneumoniae 3-fold, a longer duration of β-lactam treatment increased the risk 3.5-fold, and treatment with doses lower than those recommended clinically increased the risk nearly 6-fold [10]. The impact of short-course, high-dose amoxicillin therapy on the risk of posttreatment nasopharyngeal carriage of penicillin-resistant S. pneumoniae was evaluated in a prospective randomized clinical trial among 795 Dominican children aged 6–59 months who had respiratory tract infections [11]. Children were randomly assigned to receive amoxicillin, 90 mg/kg/day for 5 days (short-course, high-dose regimen) or 40 mg/kg/day for 10 days (standard-course regimen). There was no difference in clinical efficacy. However, nasopharyngeal specimens collected at the 28-day follow-up visit showed that, compared with children given the standard-course regimen, those treated with the short-course, high-dose regimen had a significantly lower rate of carriage of penicillin-resistant S. pneumoniae (24% vs. 32%; P = .03) and a lower risk of nonsusceptibility to trimethoprim-sulfamethoxazole (relative risk [RR], 0.77; 95% CI, 0.58–1.03; P = .08) [11]. Adherence to the prescribed treatment regimen also was significantly higher in the short-course, high-dose group (82% vs. 74%; P = .02). Additionally, among those given the standard-course regimen, adherence fell off significantly during days 6–10 compared with days 0–5 (57% vs. 79%; P < .001), mainly because the patients failed to receive sufficient medication during the latter part of therapy.
Rationale For Short-Course Therapy
Although limited data exist on the optimal duration of treatment for CAP, there is a sound scientific basis for the concept of short-course therapy [12]. Descriptions of pneumococcal pneumonia during the mid-1940s stated that antibiotics were required only during the first few hours for pneumococcal pneumonia because the initial killing of organisms occurs in the edema zone. Phagocytes observed in the first 24 h then dispose of the bacteria not destroyed by chemotherapy, assuming that the patient is immunocompetent [13].
More recently, in vitro time-kill studies have shown that if appropriate antibiotics are selected, the number of organisms can be significantly reduced within 24 h [14, 15]. Using in vitro models of infection, investigators have also shown that achieving the most effective AUC/MIC ratio for antimicrobial agents with concentration-dependent killing enhances the bactericidal effect [16].
These studies suggest that appropriate antimicrobial use can quickly reduce or eliminate the pathogen load. The clinical efficacy of this rationale was shown in a study of critically ill patients with ventilator-assisted pneumonia, finding that with appropriate antimicrobial therapy, pathogens were eradicated from the lower respiratory tract after only 3 days in 88% of patients (67/78) [17].
Contemporary Clinical Experience With Short-Course Therapy For Respiratory Infections Other Than Pneumonia
In patients with acute exacerbations of chronic bronchitis, a number of randomized, controlled, comparative trials of highly active antimicrobial agents have shown that 3- to 5-day regimens of drugs such as ceftibuten, cefdinir, dirithromycin, telithromycin, and the fluoroquinolones (e.g., levofloxacin, gemifloxacin, moxifloxacin, and gatifloxacin) are as effective as 7- or 10-day regimens of amoxicillin-clavulanic acid, cefprozil, cefuroxime axetil, clarithromycin, and levofloxacin [18–26].
A double-blind randomized trial of amoxicillin in patients with acute exacerbations of chronic bronchitis found that a high-dose regimen (3 g b.i.d.) given for 3 days was as effective as a lower-dose regimen (500 mg t.i.d.) given for 7 days [27]. In addition, several multicenter, randomized, double-blind studies of patients with acute exacerbations of chronic bronchitis have shown that the rates of clinical success (cured or improved) and bacterial eradication with a 5-day regimen of telithromycin at 800 mg once daily were comparable to those achieved with 10-day regimens of cefuroxime axetil at 500 mg b.i.d. and amoxicillin-clavulanate at 500 mg/125 mg t.i.d. [24, 25].
Clinical Experience With Short-Course Therapy For Cap
One of only a few studies before the 1990s that specifically addressed the issue of duration of therapy for uncomplicated primary pneumonia was conducted among 73 patients aged 12–60 years (mean age, 30 years) who were treated at a teaching hospital in Nigeria [13]. All patients had evidence of pneumonia on chest radiography, and the majority (65 patients) received benzylpenicillin only. Antibiotic therapy was initiated and continued until the patient was afebrile (temperature of ⩽37.2°C) for 24 h. Organisms, mainly S. pneumoniae, were identified in 42 cases by culture of sputum from 38 patients, 19 of whom were bacteremic. Antibiotics were administered for <3 days to 80% of the patients, and the average duration of therapy for the entire study population was 2.54 days. Patients were discharged from the hospital after an average of 4 days, and follow-up chest radiography showed complete resolution within an average of 25.6 days (range, 14–56 days). The investigator concluded that, in treating pneumonia, antibiotics could be stopped after the patient had been afebrile for 24 h, reducing the duration of hospitalization and exposure to antibiotic.
If temperature is used as an indicator of clinical improvement or stability, short-course antimicrobial therapy of 5 days' duration will be appropriate for the majority of patients. In a recent prospective, multicenter cohort study of 686 adults hospitalized with CAP, the median time to becoming afebrile was 2 days if defined by a temperature of 38.3°C and 3 days if defined as either 37.8°C or 37.2°C [28]. Therefore, if patients with pneumococcal pneumonia are treated for 3 days after becoming afebrile, as recommended by the IDSA, the total duration of therapy would be 5 or 6 days for most patients, depending on the definition of fever [1].
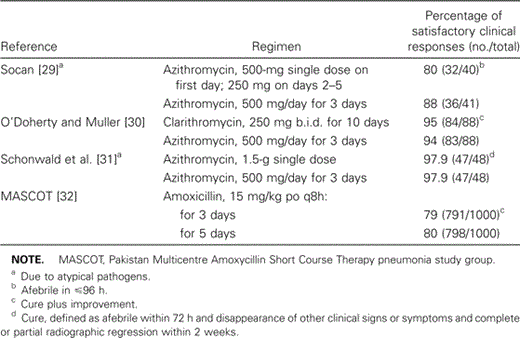
Several published studies evaluating the utility of short-course oral therapy for the treatment of CAP have involved azithromycin because of its prolonged postantibiotic effect (table 2) [29–32]. In a study of adult outpatients with mild to moderate CAP, a 3-day course of azithromycin at 500 mg once daily was as effective clinically as a 10-day course of clarithromycin at 250 mg b.i.d. [30]. Another outpatient study conducted at 7 hospitals compared the efficacy of 3- and 5-day courses of amoxicillin among 2000 children aged 2–59 months with nonsevere pneumonia [32]. Failure rates with either treatment regimen were ∼20%. Nonadherence was more common among those who received the 5-day regimen than among recipients of the 3-day regimen. In the 3-day group, 2% of the children were noncompliant after 3 days of treatment, compared with 5% of children in the 5-day group after 5 days (OR, 2.9; 95% CI, 1.6–5.1; P < .001) [32]. Moreover, nonadherence was significantly associated with treatment failure in the group treated for 5 days (P < .0001) [32].
The data described above add valuable insight into the clinical utility of short-course therapy. Certainly, enhanced microbial eradication and improved clinical outcomes are key issues in the management of all respiratory tract infections. However, a major factor limiting the “real world” success of these agents in the short-course setting is the emergence of significant antimicrobial resistance. As discussed in detail by Karchmer [33] in this supplement, penicillin-, macrolide-, fluoroquinolone-, and multidrug-resistant strains of S. pneumoniae have been shown, by several surveillance studies, to be increasing dramatically. The longest-running surveillance study, the Tracking Resistance in the United States Today study, has shown that the rates of intermediate-level and high-level resistance to penicillin, macrolides, and fluoroquinolones in S. pneumoniae have been steadily increasing since 1996, with the largest proportional increase seen in S. pneumoniae with high-level resistance [34–37].
One of the most recent US surveillance studies of the prevalence of antimicrobial resistance, the Prospective Resistant Organism Tracking and Epidemiology of the Ketolide Telithromycin–United States study, found that 38.9% of S. pneumoniae isolates collected throughout the United States were nonsusceptible to penicillin G, 31% were resistant to the macrolides, and 26.4% showed high-level resistance. The clinical relevance of β-lactam and macrolide resistance for CAP is somewhat controversial and, in part, depends on the interpretation of in vitro break points for susceptibility and resistance [38, 39]. However, increasing experience suggests that a high level of in vitro resistance can be associated with clinical failure and, therefore, is of significant concern. Although resistance to the fluoroquinolones among North American isolates is relatively low, ∼1%–2%, anecdotal reports of clinical failure due to resistance have been reported [40, 41].
The treatment for CAP is often initiated empirically before the causative organism has been identified. Antibiotic selection for empirical treatment should take into consideration the severity of illness, the likely pathogen, spectrum of activity, and local resistance patterns [1, 2, 42].
Another issue in determining the success of any antimicrobial regimen is patient compliance. In a review of the 76 studies from 1986 through 2000 that assessed patient compliance, as measured by electronic monitoring and various dosing regimens, results showed that compliance is significantly improved with once-daily dosing compared with 3- or 4-times-daily dosing (P = .008, P < .001, respectively). There was no significant difference found between once-daily and twice-daily regimens [43]. In addition, better compliance has also been associated with durations of therapy of <7 days than with longer durations of therapy [44, 45]. Short-course therapy is convenient for patients and their caregivers, and the data suggest that it improves compliance, a key determinant of therapeutic success or failure.
Clearly, the need exists for new treatment algorithms to incorporate advances in pharmacokinetically and pharmacodynamically enhanced agents with a targeted spectrum of activity against typical and atypical pathogens, including resistant strains, in a relatively short time frame with a once-daily dosing regimen. The ketolide family of antimicrobial agents has been developed for the treatment of community-acquired respiratory tract infections, including CAP [46]. Telithromycin, the first ketolide to undergo clinical development, possesses enhanced activity against penicillin- and macrolide-resistant streptococci [47, 48]. In addition, telithromycin displays marked concentration-dependent killing [49] and has an extended half-life that permits once-daily oral administration [50].
In terms of short-course therapy, a multicenter, double-blind, randomized clinical trial compared treatment with telithromycin, 800 mg once daily for 5 days or for 7 days, against treatment with clarithromycin, 500 mg b.i.d. for 10 days (results presented at an infectious disease conference held in September 2002) [51]. The clinical and bacteriologic response rates with telithromycin given for 5 days were comparable to those of clarithromycin given for 10 days (table 3). The treatments were generally well tolerated by patients during the study. Treatment-emergent adverse events considered possibly related to study medication were similar across treatment groups (24.4% [46/193] of patients treated with short-course telithromycin and 21.9% [41/187] of patients treated with the standard clarithromycin regimen). Overall, the efficacy and safety of once-daily telithromycin given for 5 days was equivalent to that of clarithromycin at 500 mg b.i.d. for 10 days for the treatment of CAP.
Telithromycin as short-course therapy for community-acquired pneumonia in a study of 581 patients by Tellier et al. [51].
In another recently reported study comparing shorter-course with “standard duration” therapy for CAP, Dunbar et al. [52] reported that a 5-day course of levofloxacin at 750 mg/day was equivalent to a 10-day course of levofloxacin at 500 mg/day.
Summary
Although well-designed, randomized controlled clinical trials to establish the optimal duration of therapy for CAP are lacking, available data indicate that short-course therapy is effective. Short-course therapy has the potential not only to improve efficacy and safety but also to minimize the evolution of resistance. Adjustments to the dosage amount, interval, and duration of antibiotics based on their known pharmacokinetic and pharmacodynamic profiles should decrease the potential for resistance. Short-course therapy is more convenient for the patient, improves compliance, decreases adverse effects, and helps slow the rise in antimicrobial resistance.
Acknowledgments
Conflict of interest. T.M.F. receives grant and/or research support from Abbott, AstraZeneca, Bayer, Bristol-Myers Squibb, Ortho-McNeil, and GlaxoSmithKline; is a consultant for Abbott, Ortho-McNeil, Aventis, GlaxoSmithKline, Pfizer, Bristol-Myers Squibb, Bayer, and Wyeth; and is a member of the speakers bureaus for Abbott, Ortho-McNeil, Merck, GlaxoSmithKline, Wyeth, and Pfizer.
References
- antibiotics
- patient compliance
- community acquired pneumonia
- drug resistance, microbial
- fluoroquinolones
- lactams
- outpatients
- respiratory system
- streptococcus pneumoniae
- macrolides
- lower respiratory tract infections
- pharmacodynamics
- pathogenic organism
- antimicrobials
- adverse event
- duration of treatment




![Telithromycin as short-course therapy for community-acquired pneumonia in a study of 581 patients by Tellier et al. [51].](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/cid/39/Supplement_3/10.1086_421354/1/m_39-Supplement_3-S159-tbl003.gif?Expires=1716322716&Signature=fjZuice6xfcCtgU0HXPswDk9cHVnhmQgWwetsA7Kzk7KvtO7I6ca1s6dDqKRGrVL0DIWHdefqcMncSV2MMMehR5YxS9vYh4E1fE20i20hiipJjplMBwNnHhCus9KWZAGtXID3V333goraLc~pplfve7BMHyLQTigMOgS9ZJWbtFVYqSDdREBpad6aG3hAB71KLcs7DaBfFRxrBqqAbFLW1nI5hssrea0DUIBgFmghM2bF4~KUk6OucjgKzI1QmuK-ji3bFja~GZo9WtrJRouwl1030v9NcFglVDRM-yD0g~AsBHf2WwgTheU9CH6Y0VqzOrX6BJN17t38qKxe0lw1w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

Comments