Abstract
Currently, limited information is available to clinicians regarding the long-term efficacy of omalizumab treatment for allergic asthma. In this report, we aimed to (i) systematically review the evidence regarding the long-term efficacy of omalizumab in patients with persistent uncontrolled allergic asthma and to (ii) discuss the cost-effectiveness evidence published for omalizumab in this patient population. A comprehensive search for randomized controlled trials (RCTs; ≥52 weeks) was performed and six studies met our final inclusion criteria (n = 2,749). Omalizumab was associated with significant improvements in quality of life and the Global Evaluation of Treatment Effectiveness. Omalizumab also allowed patients to completely withdraw from inhaled corticosteroid therapy and did not increase the overall incidence of adverse events. However, there was insufficient evidence that omalizumab reduced the incidence of exacerbations and the cost-effectiveness of omalizumab varied across studies. Our data indicated that omalizumab use for at least 52 weeks in patients with persistent uncontrolled allergic asthma was accompanied by an acceptable safety profile, but it lacked effect on the asthma exacerbations. Use of omalizumab was associated with a higher cost than conventional therapy, but these increases may be cost-effective if the medication is used in patients with severe allergic asthma.
Similar content being viewed by others
Introduction
Asthma is characterized by bronchial inflammation, airway hyper-responsiveness induced by specific and nonspecific stimuli and reversible bronchial obstruction1,2,3. An estimated 57% of these asthma patients suffer from uncontrolled asthma and a substantial proportion of severe cases are attributable to allergic immunoglobulin E (IgE)-mediated mechanisms4,5,6,7,8. Patients with persistent uncontrolled asthma are at high risk of asthma-related hospitalization and mortality, suffer significant impairments in their quality of life (QOL) and account for the majority of asthma-related costs. The Global Initiative for Asthma (GINA) guidelines recommend a stepwise approach to asthma control, with treatment being stepped up until control is achieved and maintained. However, even with the availability of these asthma guidelines and the best available treatments, approximately one third of patients continue to suffer from inadequately controlled symptoms. For patients whose asthma remains uncontrolled at this step, GINA recommends adding oral corticosteroids (OCS) or anti-IgE treatment with omalizumab9. However, adding OCS is associated with severe side effects. Specific targeting of IgE with an anti-IgE antibody therefore represents a promising approach to the treatment of allergic asthma10,11,12. Omalizumab is a recombinant humanized IgG1 monoclonal anti-IgE antibody that binds IgE at the same epitope on the Fc region that binds to the IgE receptor13,14,15.
Although omalizumab is an effective intervention as an add-on therapy in the management of severe persistent allergic asthma, important questions remain regarding the role of omalizumab in the treatment of asthma based on current guidelines. Updated National Institute for Health and Care Excellence (NICE 2013) guidelines recommend use only in patients with inadequately controlled severe persistent allergic asthma who require continuous or frequent treatments with oral corticosteroids16. However, this recommendation is not strongly supported by evidence. Indeed, other international guidelines are less proscriptive and recommend this treatment for patients who remain suboptimally controlled after maximal therapy with inhaled corticosteroids (ICS) plus long-acting beta2-agonists (LABA), as well as a third controller (e.g., leukotriene antagonists or theophyllines)16. Furthermore, evidence is somewhat lacking regarding the efficacy of this drug in patients with more severe asthma, as many trials include participants with mild or moderate disease16. In the US, omalizumab is recommended for the treatment of adults and adolescents (aged 12 years and above) with moderate-to-severe allergic asthma that is inadequately controlled in spite of treatment with ICS. This approval was based on previous pivotal clinical trials that did not include patients using LABAs, as these trials were designed and implemented at a time when LABAs were not the standard of care for asthma. Over time, LABAs have become the standard of care for patients with asthma that is not adequately controlled with ICS therapy17. The updated asthma treatment guidelines recommend omalizumab as an add-on treatment for steps 5 and 6 and include high doses of ICS and LABA combination therapy (with OCS added at step 6). However, little evidence has been found for this recommendation9,18. Omalizumab treatment efficacy is often evaluated at 16 weeks; however, in many patients, an extension of treatment is essential to improve symptoms, medication use, lung function and quality of life outcomes. For this reason, when to stop omalizumab therapy, as well as its long-term effects, are unclear. Long-term studies will be needed to clarify these issues. In 2009, the US Food and Drug Administration (FDA) raised concerns about the incidence of adverse cardiovascular and cerebrovascular events in the omalizumab treatment group of the EXCELS study19. Such events were not described in previous analyses of clinical data and several systematic reviews have not observed increased cardiovascular risk among patients taking omalizumab in studies shorter than 1 year16,17,20,21,22. The FDA is not recommending any changes in the drug's prescription information at this time. The long-term (more than 1 year) efficacy and safety of omalizumab remain a concern.
Numerous randomized clinical trials (RCTs) have demonstrated omalizumab's efficacy in patients with moderate-to-severe allergic asthma, but treatment periods have always been relatively short in these trials (mean: 28 [range: 20 to 32] weeks). We have summarized the main characteristics of those studies in supplemental table 123,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42. Because the value of short-term treatment outcomes is relatively limited, assessments covering longer periods of treatment are necessary. As asthma is a chronic disease, long-term studies are necessary to evaluate the effects of omalizumab therapy, especially in children. There is a lack of robust evidence regarding the efficacy of omalizumab beyond 52 weeks in both adults and children. In recent years, several RCTs have assessed the effects of long-term (≥52 weeks) omalizumab treatment in patients with allergic asthma. However, the evidence is inadequate for drawing robust conclusions, because the sample sizes of these studies were relatively modest and their conclusions were inconsistent. To comprehensively evaluate the evidence relating to these issues, we conducted this study to determine whether omalizumab is safe and effective when used for more than 52 weeks in patients with persistent, uncontrolled, moderate-to-severe allergic asthma in spite of high-dose ICS or ICS plus LABA and to provide clinicians with evidence regarding the long-term efficiency of omalizumab treatment in patients with allergic asthma. Additionally, omalizumab is more expensive than other asthma treatments and evidence of economic benefits for patients and reimbursement authorities remains in demand43. Therefore, the cost-effectiveness evidence published for omalizumab in this patient population was also examined.
Results
Characteristics of the studies
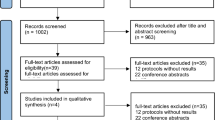
The electronic database search identified 2,354 citations. Of these, the first screening excluded 2,088 citations based on abstracts or titles, leaving 266 articles for full-text review. Of these articles, 236 studies were excluded because they contained no relative outcomes or were non-randomized, or non-placebo controlled studies. Following a more detailed review, fifteen short term trials (<52 weeks) were excluded. Finally, six studies were included in our systematic review and meta-analysis44,45,46,47,48,49. The detailed steps of the study selection process are shown in Figure 1.
The primary characteristics of the included studies are summarized in Table 1. A total of 2,749 participants with allergic asthma were included in these studies. Patients with severe asthma were recruited in three studies44,45,46 and patients with moderate-to- severe asthma were recruited in three additional studies47,48,49. Mean baseline FEV1 values varied from 64.1 to 92.9% of predicted. Durations of treatment ranged from 52 to 62 weeks. Four studies included adolescents and adults only44,45,46,47 and two also included pediatric paticipants48,49. Regarding the risk of bias, only one study met all five accepted criteria48 (Table 2).
Treatment effectiveness and safety
The overall designs of these studies were as follows: after a run-in phase (4–8 weeks), omalizumab was administrated as an adjunctive therapy to inhaled or oral corticosteroids for 16 to 28 weeks (stable steroid phase), followed by a steroid-reduction phase of 12 to 28 additional weeks, during which doses were decreased only if patients met strict criteria for steroid reduction. We double-counted two end points (stable steroid phase and steroid-reduction phase) and using these single primary efficacy endpoints (end of the steroid-reduction phase), included the rates of clinically significant asthma exacerbations, reductions in ICS doses, Global Evaluation of Treatment Effectiveness (GETE), Asthma Quality of Life Questionnaire (AQLQ), asthma symptom scores, lung function and adverse events (AEs), over a period of 52 weeks. Although all studies included a steroid reduction phase, only two reported data regarding a stable steroid phase44,48. The data showed that omalizumab-treated patients experienced significantly lower rates of clinically significant asthma exacerbations compared with patients who received a placebo during the stable phase (0.45 vs 0.64; p = 0.007) and the relative risk (RR) was 0.69 [0.53, 0.90]. Data from the studies with a steroid-reduction phase demonstrated reductions in exacerbation rates that remained significant over periods of 52 weeks (RR 0.63, 95% CI [0.55, 0.71]; p < 0.0001) (Figure 2). Statistical heterogeneity was not observed (I2 = 0%, p = 0.46). During the steroid-reduction phase, ICS doses were significantly decreased in omalizumab-treated patients compared with the placebo group (RR 1.86, 95% CI [1.51, 2.29]; p < 0.0001). Heterogeneity was not observed (I2 = 0%, p = 0.47). At 52 weeks, both GETE (an excellent or good response) and AQLQ scores (≥1.5 points from baseline) favored omalizumab (RR1.54, 95%CI [1.38, 1.72]; p < 0.00001 and RR 2.08, 95% CI [1.03, 4.20]; p = 0.04 respectively) (table 3). Four studies assessed adverse events (AEs) and omalizumab was well tolerated45,47,48,49. Common adverse events included the following: lower respiratory tract infection, nasopharyngitis, headache, injection site pain, injection site reaction and arthralgia. Based on the results of the meta-analysis, the numbers of patients reporting AEs was similar in both treatment groups (RR 0.97, 95% CI [0.93, 1.01]; p = 0.11). Statistical heterogeneity was not observed (I2 = 3%, p = 0.38). Serious adverse events, such as death, asthma exacerbation, pruritus, acute appendicitis, sphenoid sinusitis, intestinal obstruction and mild chest pain were reported. However, none of these was considered drug-related. The incidence and profile of serious adverse events were slightly lower in the omalizumab group (RR 0.55, 95% CI [0.37, 0.82]; p = 0.003). Statistical heterogeneity was not observed (I2 = 0%, p = 0.70) (Figure 3). No clinically relevant abnormalities in laboratory tests (including platelet count) were observed.
With regard to asthma symptoms and lung function, descriptive analysis methods were utilized, as most of these data were unavailable or unsuitable for analysis. Two RCTs demonstrated greater reductions in asthma symptom scores than placebo46,48. However, the effects of omalizumab on lung function were discrepant45,46,47,49. Only one RCT demonstrated that pulmonary function (FEV1) was significantly better in the omalizumab group than in the control group46 (Supplemental table 2).
The cost-effectiveness of omalizumab add-on therapy has been assessed in several analyses43,50,51,52,53,54,55,56,57. Marked variations were noted across studies regarding cost-effectiveness (Table 4). Campbell et al. concluded that adding omalizumab improves quality-adjusted life years (QALY), with increased direct medical costs51. Their findings also suggested that cost-effectiveness improves when 16-week assessments to determine responses are used to guide decisions regarding long-term treatment. Dal Negro et al. concluded that omalizumab improves health-related quality of life but also substantially increases costs53. Nooten and Wu et al. reported that omalizumab was not cost-effective and noted incremental cost-effectiveness ratios (ICERs) of [euro]38,371 and $821,00054,56. Data from the real-life 1-year randomized open-label study (ETOPA), using Canada as a reference country, noted an ICER of [euro]31,209 in patients with severe persistent allergic asthma50. Devilde and Oba et al. concluded that omalizumab was cost-effective for patients with severe allergic asthma52,55. This finding suggests that asthma severity and the risk of asthma exacerbations should be considered when determining the cost-effectiveness of omalizumab.
Sensitivity analysis and publication bias
Two outcomes (asthma exacerbations and AQLQ) were analyzed in an open label study46. When only randomized, double-blind trials were evaluated44,45,47,48,49, there were no significant differences in the incidences of asthma exacerbations (RR 0.63, 95% CI [0.54, 0.73]; p < 0.00001). AQLQ was also not significantly altered when an open label study was excluded (RR1.57, 95% CI [1.23, 2.01]; p = 0.0003). Other sensitivity and subgroup analyses are summarized in table 4. A sensitivity analysis revealed that the conclusions of the meta-analysis remained robust regarding methodological changes, indicating that the results of our study are believable and reliable. Publication bias was detected by Begg's and Egger's tests. Funnel plots of the four studies evaluating the effects of omalizumab on asthma exacerbation appeared to be symmetrical upon visual examination. The data suggested that there was no evidence of publication bias (Begg's test, p = 0.373, Eger's test, p = 0.568).
Discussion
Severe persistent asthma remains poorly understood and difficult to manage. Previous studies and reviews have demonstrated that omalizumab is an effective treatment option for moderate to severe allergic asthma. However, evidence regarding its long-term (beyond 52 weeks) efficacy and safety in both adults and children is very limited. In recent years, new randomized trials have assessed the efficacy and safety of omalizumab beyond 52 weeks in both adults and children with allergic asthma that is poorly controlled in spite of treatment with high doses of ICS or ICS plus LABA. Therefore, it seems reasonable to explore this issue further. In contrast to previous systematic reviews that included studies of short duration (less than 1 year), we included only long-term trials involving patients with persistent uncontrolled allergic asthma to assess the efficacy of and risk associated with omalizumab. Based on the pooled analyses, we found that omalizumab significantly reduced the incidence of asthma exacerbations and ICS use and improved scores on the GETE and AQLQ, compared with control subjects. Additionally, omalizumab was well tolerated and demonstrated an acceptable safety profile. Costs also increased, but the drug may be cost-effective if used in patients with severe allergic asthma.
Severe asthma exacerbations are a major concern, as they are responsible for the mortality associated with asthma and contribute significantly to the health costs of the disease58. Indeed, decreasing the rate of asthma exacerbations is a key goal of asthma management and is likely to be associated with improvements in asthma-related quality of life and reductions in the burdens imposed on patients and health care systems. Our meta-analysis demonstrated that compared with the control group, a significant reduction was observed in the rate of exacerbation for patients receiving omalizumab add-on treatment. However, there was some degree of heterogeneity in the definition of exacerbations within trials (Table 1), which may influence the efficacy of omalizumab on asthma exacerbations. Moreover, in placebo controlled study if you reduce treatment you will see more exacerbations allied to treatment dose reduction (e.g., steroid, LABA or a third controller). Therefore, the results should be interpreted cautiously due to these limitations. In other words, a lack of robust evidence existed that omalizumab reduced exacerbations in allergic asthma patients who were uncontrolled by the best available therapy. Our analyses demonstrated that the safety profile of omalizumab was excellent and the treatment was well tolerated, as only infrequent and generally mild local reactions were observed following treatment. There were no drug-related serious adverse events. ICS are anti-inflammatory medications that inhibit inflammatory cell migration and activation, reduce airway hyperresponsiveness and block late phase reactions to allergens44. ICS are fundamental in the treatment of asthma and are well tolerated and safe when administered at recommended dosages. In our meta-analysis, omalizumab-treated patients were more likely to be completely withdrawn from corticosteroid therapy. Reductions in ICS doses were achieved without precipitating worsening symptoms, increasing the use of rescue medications, altering lung function, or causing asthma exacerbations.
The GETE is a composite measure that includes patient interviews, reviews of medical notes, spirometry and diaries of symptoms, rescue medication use and peak expiratory flow (PEF) values59. Our result demonstrated that more patients in the omalizumab group were rated as excellent or good compared with the control group by this measure. The emotional, physical and social aspects of the daily lives of patients with persistent uncontrolled allergic asthma are significantly impaired3. In our study, improvements in AQLQ overall scores (symptoms, activities, environment and emotions) occurred in a larger proportion of patients receiving add-on omalizumab therapy, as these patients experienced significant (≥1.5-points) improvements in asthma-related QOL compared with the control group. This result was consistent with those of previous meta-analyses completed by Chipps et al. and Niebauer et al. (both in 2006)60,61.
Our study also demonstrated significant improvements in asthma symptom scores for omalizumab treatment compared with placebo. However, the effects of omalizumab on lung function were inconsistent. Several studies indicated that lung function parameters were not sensitive enough to mirror the treatment effectiveness of omalizumab in patients with asthma45,46,47,49. The improvements in lung function with omalizumab were consistent with its anti-inflammatory effects, improvements that may be attributed to suppressed free IgE levels62,63. The effects of omalizumab on lung function were particularly notable when placebo-treated patients received higher doses of long-acting bronchodilators, as they would be expected to diminish or eliminate any differences between the groups regarding lung function62. Seven studies were considered in this review and provided evidence of the cost-effectiveness of add-on omalizumab in patients with allergic asthma that was poorly controlled in spite of high doses of ICS or ICS plus LABAs. Although the findings of some economic analyses of omalizumab are unfavorable, there are published cost-effectiveness analyses that demonstrate that omalizumab is cost-effective in patients with inadequately controlled severe allergic asthma. Based on the high cost of omalizumab, it is important to determine which patients benefit most from its use.
There are some limitations that need to be considered. First, we included an open-label study under the assumption that real-world effectiveness data are meaningful and avoid the biases inherent in selecting studies. Although not as scientifically rigorous as double-blind trials, these types of trials remain important in understanding how a therapy performs in a setting more reflective of the real world46. However, the limitations of such trial designs (e.g., the potential for bias in the assessment of outcomes) should be considered when interpreting open-label data, which should be considered in the context of other randomized, double-blind data. To reconcile these issues, a sensitivity analysis was conducted whereby the meta-analysis was reanalyzed, excluding this open label study, as described in previous studies64,65,66. The sensitivity analysis determined that the conclusions remained robust for methodological changes, demonstrating that the data of present study are reliable. Second, some forms of detailed information (e.g., lung function and rescue medications) were unavailable in most studies, which prevented us conducting more detailed and relevant analyses and obtaining more comprehensive results. Therefore, the effects of omalizumab on lung function and rescue medications warrant further investigation.
The major strength of our study was that we included only long-term trials that provided evidence regarding the long-term efficacy and safety of omalizumab treatment in patients with uncontrolled persistent allergic asthma. Our study is in line with current guidelines, which recommend that omalizumab be considered as part of steps 5 and 6 of the stepwise treatment for patients with persistent allergic asthma that is uncontrolled in spite of treatment with high-dose ICS or ICS plus LABAs and/or a third controller (including OCS)9. Previous Cochrane reviews have consistently observed that omalizumab is both effective and safe in patients with inadequately controlled persistent asthma compared with conventional therapy16,20 and this conclusion is strengthened by our findings.
In summary, our findings have the following potential regulatory and clinical implications: 1) the use of omalizumab for at least 52 weeks in severe asthmatic patients is effective and is accompanied by an acceptable safety profile; 2) subgroup analyses provided further evidence for the current asthma guideline recommendations to consider omalizumab in steps 5 or 6 for patients with persistent allergic asthma that remains uncontrolled in spite of treatment with high-dose ICS plus LABAs and/or a third controller (including OCS); 3) Although omalizumab is often prescribed to reduce exacerbations, it lacks effect on exacerbations in patients with persistent uncontrolled allergic asthma; 4) costs increased, but the use of omalizumab could be cost-effective if the drug is used to treat patients with severe allergic asthma. However, the evidence in children is weaker and more ambiguous. Further studies are necessary to answer several practical questions, including how and when to reduce or stop treatment and how to identify possible genetic or biochemical markers that can predict treatment responses.
Methods
Data Sources and Search Strategy
We searched Medline, the Cochrane Database of Systematic Reviews (CDSR), EMBASE databases, the Cochrane Central Register of Controlled Trials (CENTRAL), the National Institutes for Health (NIH) ClinicalTrials.gov Register, Current Controlled Trials and the FDA (www.fda.gov) database, which include all papers published up until March 2014, using the following search terms: “anti-immunoglobulin E” or “anti-IgE” or “Omalizumab” or “Xolair” and “asthma”. Trials were not excluded on the basis of language. All eligible studies were retrieved and their reference lists were checked for additional articles. To ensure a complete review of the available studies, the abstracts of relevant scientific meetings were also examined. We also made efforts to contact authors in cases where relevant data were unclear. Trials published solely in abstract form were excluded.
Selection criteria
Specific inclusion criteria were as follows: (1) adults/adolescents (12 years or older) and children (aged between 6 and 12 years) with a diagnosis of persistent uncontrolled moderate to severe allergic asthma in spite of high-dose ICS or ICS plus LABAs, (2) investigations of patients who received subcutaneous omalizumab therapy at any dose as a guidelines-based therapy, (3) randomized (parallel group) placebo-controlled trials and (4) RCTs that reported the following outcomes: asthma exacerbations, inhaled corticosteroid (ICS) use, Evaluation of Treatment Effectiveness (GETE), QOL, asthma symptoms, lung function, rescue medication and adverse events. An exacerbation was defined as a worsening of asthma symptoms requiring treatment with systemic corticosteroids and increased doses of rescue medication, hospitalization or an emergency room visit, or an unscheduled physician visit. Short term trials (<52 weeks) were excluded.
Data extraction
This systematic review was undertaken according to PRISMA guidelines67. Titles and abstracts were independently reviewed by two reviewers (T.L. and C.C.) to determine their potential relevance. Data from all studies included in this analysis were obtained during the end of the extension phases of the trial. Any disagreements were resolved by consensus with a third reviewer when necessary. In the case of unpublished reports or multiple publications, data from the most recent version were extracted. After obtaining full-text, the authors independently assessed all studies for inclusion based on the predefined criteria. If studies had partly overlapping subjects, the study with the larger sample size was selected. The quality of each trial was evaluated using the Cochrane five risk of bias domains tool.
Data analysis
RR and 95% CIs were used to analyze the efficacy and safety of omalizumab as an add-on therapy for persistent uncontrolled allergic asthma. Heterogeneity assumptions were assessed using the I2 statistic. I2 values of 25%, 50% and 75% represented low, moderate and high heterogeneity, respectively68. When heterogeneity was noted, subgroup analyses were performed to seek out the source of the heterogeneity. Studies of poorer methodological quality, such as unblinded or open-label trials, may have exhibited exaggerated treatment effects. Excluding them may have resulted in increased internal validity but may also have reduced the external validity of the analysis64,65,66. To reconcile these issues, separate subgroup and sensitivity analyses were conducted, whereby the meta-analysis was reanalyzed, including risks of bias (low vs high), ages of patients (children vs adults and adolescents), asthma severity (severe vs moderate to severe) and intervention (omalizumab/ICS vs omalizumab/ICS + LABA), using a Mantel-Haenszel fixed-effects model and excluding unblinded or open label studies. Publication bias was determined using the funnel plot and assessed by Egger's test69. All analyses were performed with Review Manager (Version 5.0.1, The Cochrane Collaboration) and Stata (Version 10.0, Stata Corporation, USA). A p-value of <0.05 was considered to be statistically significant.
Change history
14 August 2015
A correction has been published and is appended to both the HTML and PDF versions of this paper. The error has not been fixed in the paper.
References
Peters, S. P. et al. Real-world evaluation of asthma control and treatment (REACT): findings from a national web-based survey. J Allergy Clin Immunol 119, 1454–1461 (2007).
Rijavec, M., Korošec, P., Zavbi, M., Kern, I. & Malovrh, M. M. Let-7a is differentially expressed in bronchial biopsies of patients with severe asthma. Sci Rep 4, 6103 (2014).
Bardelas, J., Figliomeni, M., Kianifard, F. & Meng, X. A 26-Week, Randomized, Double-Blind, Placebo-Controlled, Multicenter Study to Evaluate the Effect of Omalizumab on Asthma Control in Patients with Persistent Allergic Asthma. J Asthma 49, 144–152 (2012).
Ayres, J. G. et al. Efficacy and tolerability of anti-immunoglobulin E therapy with omalizumab in patients with poorly controlled (moderate-to-severe) allergic asthma. Allergy 59, 701–708 (2004).
Buhl, R. et al. The anti-IgE antibody omalizumab improves asthma-related quality of life in patients with allergic asthma. Eur Respir J 20, 1088–1094 (2002).
Luskin, A. T., Kosinski, M., Bresnahan, B. W., Ashby, M. & Wong, D. A. Symptom control and improved functioning: the effect of omalizumab on asthma-related quality of life (ARQL). J Asthma 42, 823–827 (2005).
Karpel, J. et al. Effectiveness of omalizumab in reducing corticosteroid burden in patients with moderate to severe persistent allergic asthma. Ann Allergy Asthma Immunol 105, 465–470 (2010).
Kulus, M. et al. Omalizumab in children with inadequately controlled severe allergic (IgE-mediated) asthma. Curr Med Res Opin 26, 1285–1293 (2010).
National Heart Lung and Blood Institute, Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma - Summary Report 2007. <http://www.nhlbi.nih.gov/health-pro/guidelines/current/asthma-guidelines/summary-report-2007.htm> (2007) Date of access: 20/05/2014.
Noga, O., Hanf, G. & Kunkel, G. Immunological and clinical changes in allergic asthmatics following treatment with omalizumab. Int Arch Allergy Immunol 131, 46–52 (2003).
Berger, W., Gupta, N., McAlary, M. & Fowler-Taylor, A. Evaluation of long-term safety of the anti-IgE antibody, omalizumab, in children with allergic asthma. Ann Allergy Asthma Immunol 91, 182–188 (2003).
Silkoff, P. E., Romero, F. A., Gupta, N., Townley, R. G. & Milgrom, H. Exhaled nitric oxide in children with asthma receiving Xolair (omalizumab), a monoclonal anti-immunoglobulin E antibody. Pediatrics 113, e308–12 (2004).
Platts-Mills, T. A. The role of immunoglobulin E in allergy and asthma. Am J Respir Crit Care Med 164, S1–5 (2001).
Bousquet, J. et al. Predicting response to omalizumab, an anti-IgE antibody, in patients with allergic asthma. Chest 125, 1378–1386 (2004).
Hochhaus, G. et al. Pharmacodynamics of omalizumab: implications for optimised dosing strategies and clinical efficacy in the treatment of allergic asthma. Curr Med Res Opin 19, 491–498 (2003).
Normansell, R., Walker, S., Milan, S. J., Walters, E. H. & Nair, P. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev 13, 1, CD003559 (2014).
Chipps, B. E., Figliomeni, M. & Spector, S. Omalizumab: an update on efficacy and safety in moderate-to-severe allergic asthma. Allergy Asthma Proc 33, 377–385 (2012).
National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the diagnosis and management of asthma. <http://www.ncbi.nlm.nih.gov/books/NBK7232/> (2007) Date of access: 20/07/2014.
Vieira, T., de Oliveira, J. F. & da Graça Castel-Branco, M. Short and long-term quality of life and asthma control with omalizumab therapy in a real life setting in Portugal. Allergol Immunopathol (Madr) 42, 3–10 (2014).
Walker, S., Monteil, M., Phelan, K., Lasserson, T. J. & Walters, E. H. Anti-IgE for chronic asthma in adults and children. Cochrane Database Syst Rev 19, CD003559 (2006).
Corren, J. et al. Safety and tolerability of omalizumab. Clin Esp Allergy 39, 788–797 (2009).
Rodrigo, G. J., Neffen, H. & Castro-Rodriguez, J. A. Efficacy and safety of subcutaneous omalizumab vs Placebo as add-on therapy to corticosteroids for children and adults with asthma: a systematic review. Chest 139, 28–35 (2011).
Bardelas, J., Figliomeni, M., Kianifard, F. & Meng, X. A 26-week, randomized, double-blind, placebo-controlled, multicenter study to evaluate the effect of omalizumab on asthma control in patients with persistent allergic asthma. J Asthma 49, 44–52 (2012).
Bousquet, J. et al. Persistency of response to omalizumab therapy in severe allergic (IgE-mediated) asthma. Allergy 66, 671–678 (2011).
Busse, W. et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol 108, 184–190 (2001).
Chanez, P. et al. Omalizumab-induced decrease of FcξRI expression in patients with severe allergic asthma. Respir Med 104, 1608–1617 (2010).
Hanania, N. A. et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Ann Intern Med 154, 573–582 (2011).
Holgate, S. T. et al. Efficacy and safety of a recombinant anti-immunoglobulin E antibody (omalizumab) in severe allergic asthma. Clin Exp Allergy 34, 632–638 (2004).
Hoshino, M. & Ohtawa, J. Effects of adding omalizumab, an anti-immunoglobulin E antibody, on airway wall thickening in asthma. Respiration 83, 520–528 (2012).
Humbert, M. et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy 60, 309–316 (2005).
Kopp, M. V. et al. Combination of omalizumab and specific immunotherapy is superior to immunotherapy in patients with seasonal allergic rhinoconjunctivitis and co-morbid seasonal allergic asthma. Clin Exp Allergy 39, 271–279 (2009).
Lemanske, R. F., Jr et al. Omalizumab improves asthma-related quality of life in children with allergic asthma. Pediatrics 110, e55 (2002).
Massanari, M., Kianifard, F., Zeldin, R. K. & Geba, G. P. Efficacy of omalizumab in cat-allergic patients with moderate-to-severe persistent asthma. Allergy Asthma Proc 30, 534–539 (2009).
Massanari, M. et al. Effect of pretreatment with omalizumab on the tolerability of specific immunotherapy in allergic asthma. J Allergy Clin Immunol 125, 383–389 (2010).
Milgrom, H. et al. Treatment of childhood asthma with anti-immunoglobulin E antibody (omalizumab). Pediatrics 108, E36 (2001).
Milgrom, H., Fowler-Taylor, A., Vidaurre, C. F. & Jayawardene, S. Safety and tolerability of omalizumab in children with allergic (IgE-mediated) asthma. Curr Med Res Opin 27, 163–169 (2011).
Ohta, K. et al. Efficacy and safety of omalizumab in an Asian population with moderate-to-severe persistent asthma. Respirology 14, 1156–1165 (2009).
Rubin, A. S. et al. Effect of omalizumab as add-on therapy on asthma-related quality of life in severe allergic asthma: a Brazilian study (QUALITX). J Asthma 49, 288–293 (2012).
Siergiejko, Z. et al. Oral corticosteroid sparing with omalizumab in severe allergic (IgE-mediated) asthma patients. Curr Med Res Opin 27, 2223–2228 (2011).
Solèr, M. et al. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J 18, 254–261 (2001).
Vignola, A. M. et al. Efficacy and tolerability of anti-immunoglobulin E therapy with omalizumab in patients with concomitant allergic asthma and persistent allergic rhinitis: SOLAR. Allergy 59, 709–717 (2004).
Zielen, S. et al. Omalizumab protects against allergen- induced bronchoconstrict-ion in allergic (immunoglobulin E-mediated) asthma. Int Arch Allergy Immunol 160, 102–110 (2013).
Sullivan, S. D. & Turk, F. An evaluation of the cost-effectiveness of omalizumab for the treatment of severe allergic asthma. Allergy 63, 670–684 (2008).
Finn, A. et al. Omalizumab improves asthma-related quality of life in patients with severe allergic asthma. J Allergy Clin Immunol 111, 278–284 (2003).
Lanier, B. Q. et al. Omalizumab is effective in the long-term control of severe allergic asthma. Ann Allergy Asthma Immunol 91, 154–159 (2003).
Niven, R., Chung, K. F., Panahloo, Z., Blogg, M. & Ayre, G. Effectiveness of omalizumab in patients with inadequately controlled severe persistent allergic asthma: an open-label study. Respir Med 102, 1371–1378 (2008).
Buhl, R. et al. Omalizumab provides long-term control in patients with moderate-to-severe allergic asthma. Eur Respir J 20, 73–78 (2002).
Lanier, B. et al. Omalizumab for the treatment of exacerbations in children with inadequately controlled allergic (IgE-mediated) asthma. J Allergy Clin Immunol 124, 1210–1216 (2009).
Busse, W. W. et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med 364, 1005–1015 (2011).
Brown, R., Turk, F., Dale, P. & Bousquet, J. Cost-effectiveness of omalizumab in patients with severe persistent allergic asthma. Allergy 62, 149–153 (2007).
Campbell, J. D., Spackman, D. E. & Sullivan, S. D. The costs and consequences of omalizumab in uncontrolled asthma from a USA payer perspective. Allergy 65, 1141–1148 (2010).
Dewilde, S., Turk, F., Tambour, M. & Sandström, T. The economic value of anti-IgE in severe persistent, IgE-mediated (allergic) asthma patients: adaptation of INNOVATE to Sweden. Curr Med Res Opin 22, 1765–1776 (2006).
Dal Negro, R. W., Pradelli, L., Tognella, S., Micheletto, C. & Iannazzo, S. Cost-utility of add-on omalizumab in difficult-to-treat allergic asthma in Italy. Eur Ann Allergy Clin Immunol 43, 45–53 (2011).
van Nooten, F. et al. Cost-effectiveness of omalizumab for uncontrolled allergic asthma in the Netherlands. J Med Econ 16, 342–348 (2012).
Oba, Y. & Salzman, G. A. Cost-effectiveness analysis of omalizumab in adults and adolescents with moderate-to-severe allergic asthma. J Allergy Clin Immunol 114, 265–269 (2004).
Wu, A. C., Paltiel, A. D., Kuntz, K. M., Weiss, S. T. & Fuhlbrigge, A. L. Cost-effectiveness of omalizumab in adults with severe asthma: results from the Asthma Policy Model. J Allergy Clin Immunol 20, 1146–1152 (2007).
Norman, G. et al. Omalizumab for the treatment of severe persistent allergic asthma: a systematic review and economic evaluation. Health Technol Assess 17, 1–342 (2013).
Bousquet, J. et al. The effect of treatment with omalizumab, an anti-IgE antibody, on asthma exacerbations and emergency medical visits in patients with severe persistent asthma. Allergy 60, 302–308 (2005).
Bousquet, J. et al. Predicting and evaluating response to omalizumab in patients with severe allergic asthma. Respir Med 101, 1483–1492 ( 2007).
Niebauer, K., Dewilde, S., Fox-Rushby, J. & Revicki, D. A. Impact of omalizumab on quality-of-life outcomes in patients with moderate-to-severe allergic asthma. Ann Allergy Asthma Immunol 96, 316–26 (2006).
Chipps, B. et al. Improvement in quality of life with omalizumab in patients with severe allergic asthma. Curr Med Res Opin 22, 2201–2208 (2006).
Owen, C. E. Anti-immunoglobulin E therapy for asthma. Pulm Pharmacol Ther 15, 417–424 (2002).
Lanier, B. Q. et al. Omalizumab is effective in the long-term control of severe allergic asthma. Ann Allergy Asthma Immunol 91,154–159 (2003).
Li, Z. & Begg, C. B. Random Effects Models for Combining Results from Controlled and Uncontrolled Studies in a Meta-Analysis. American Statistical Association 89, 1523–1527 (1994).
Dale, K. M., Coleman, C., Henyan, N. N., Kluger, J. & White, C. M. Statins and cancer risk: a meta-analysis. JAMA 295, 74–80 (2006).
Phung, O. J., Makanji, S. S., White, C. M. & Coleman, C. I. Almonds have a neutral effect on serum lipid profiles: a meta-analysis of randomized trials. J Am Diet Assoc 109, 865–873 (2009).
Liberati, A. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 151, W65–W94 (2009).
Higgins, J. P., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. BMJ 327, 557–560 (2003).
Egger, M., Davey Smith, G., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 (1997).
Acknowledgements
We would like to acknowledge our funding source: the Key Program of National Natural Science Foundation of China (No.81370126), the National Key Technologies R&D Program for the 12th Five-year Plan (2012BAI05B01) and project from the Chinese National Clinical Research Center for Respiratory Disease.
Author information
Authors and Affiliations
Contributions
W.-T.L., B.-S.W. and W.-Z.X. have contributed to the design of the study, analysis and interpretation of data and drafting a part of manuscript. C.Z., Y.H. and C.C. searched the related papers and extracted data. M.-S.Y. and W.L. carried out statistical analysis and revised manuscript. Z.C. and Y.Z. prepared all figures. B.W. and H.-H.S. designed this study and revised manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Lai, T., Wang, S., Xu, Z. et al. Long-term efficacy and safety of omalizumab in patients with persistent uncontrolled allergic asthma: a systematic review and meta-analysis. Sci Rep 5, 8191 (2015). https://doi.org/10.1038/srep08191
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep08191
This article is cited by
-
Real-world effectiveness of omalizumab for severe allergic asthma treatment in Colombia
BMC Pulmonary Medicine (2022)
-
The human viral challenge model: accelerating the evaluation of respiratory antivirals, vaccines and novel diagnostics
Respiratory Research (2018)
-
Pediatric severe asthma: a case series report and perspectives on anti-IgE treatment
BMC Pediatrics (2018)
-
Biologic agents for severe asthma patients: clinical perspectives and implications
Internal and Emergency Medicine (2018)
-
Asthma biomarkers in the age of biologics
Allergy, Asthma & Clinical Immunology (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.