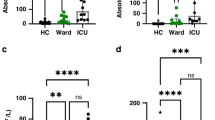
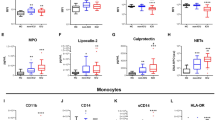
Abstract
Granulocyte colony-stimulating factor (GM-CSF), produced by CD4+ T cells, has recently been implicated in the pathogenesis of inflammatory diseases, such as multiple sclerosis and juvenile arthritis. However, the role of GM-CSF-producing CD4+ T cells in sepsis remains unknown. This study reports peripheral changes in GM-CSF-producing CD4+ T cells in septic patients and the possible underlying mechanism by which GM-CSF influences the outcome of sepsis. Forty-three septic patients, 20 SIRS patients, and 20 healthy controls were enrolled in this study and followed for 28 days to assess mortality. We measured the peripheral frequency of GM-CSF+CD4+ T cells and recorded their associated relationship with disease progression. Our data demonstrated that peripheral GM-CSF-producing CD4+ T cells were significantly higher in septic patients than in both SIRS patients and healthy controls. These cells exhibit a memory phenotype and impaired IFN-γ-secreting capacity in sepsis patients. Using a receiver operating curve analysis with 8.01% as a cut-off point, the percentage of GM-CSF+CD4+ T cells could predict the outcome of septic patients. Combined with the increase in GM-CSF-producing CD4+ T cells, inflammatory cytokines IL-1β and IL-6 were also upregulated. Using an in vitro neutrophil model, we found that GM-CSF inhibited C3aR expression, while inducing IL-8 production. Furthermore, this effect was transferrable in plasma from sepsis patients and was attenuated by inhibition of GM-CSF using an anti-GM-CSF antibody. These results indicate that GM-CSF-producing CD4+ T cells may serve as a marker of sepsis severity. Thus, targeting GM-CSF overproduction may benefit sepsis patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Levy, M. M. et al. SCCM/ESICM/ACCP/ATS/SI:2001SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit. Care Med. 31, 1250–1256 (2003).
Balk, R. A. Severe sepsis and septic shock. Crit. Care Clin. 16, 179–192 (2000).
Van Nieuwenhuijze, A. et al. GM-CSF as a therapeutic target in inflammatory diseases. Mol. Immunol. 56, 675–682 (2013).
Codarri, L. et al. RORγt derives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat. Immunol. 12, 560–567 (2011).
EI-Behi, M. et al. The encephalitogenicity of TH17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat. Immunol. 12, 568–575 (2011).
Meisel, C. et al. Granulocyte–macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression. Am. J. Respir. Crit. Care. Med. 180, 640–648 (2009).
Orozco, H. et al. Molgramostim (GM-CSF) associated with antibiotic treatment in nontraumatic abdominal sepsis. Arch. Surg. 141, 150–153 (2006).
Xu, R., Lin, F., Bao, C. & Wang, F. S. Mechanism of C5a-induced immunologic derangement in sepsis. Cell Mol. Immunol. 14, 792–793 (2017).
Burgess, A. W., Camakaris, J. & Metcalf, D. Purification and properties of colony-stimulating factor from mouse lung-conditioned medium. J. Biol. Chem. 252, 1998–2003 (1977).
Zhang, J. et al. A novel subset of helper T cells promotes immuneresponses by secreting GM-CSF. Cell Death Differ. 20, 1731–1741 (2013).
Rasouli, J. et al. Expression of GM-CSF in T cells is increased in multiple sclerosis and suppressed by IFN-β therapy. J. Immunol. 194, 5085–5093 (2015).
Luken, J. R., Barr, M. J., Chaplin, D. D., Chi, H. & Lanneganti, T. D. Inflammasome-derived IL-1β regulates the production of GM-CSF by CD4(+) T cells and γδT cells. J. Immunol. 188, 3107–3115 (2012).
Hazelzet, J. A. et al. Complement activation in relation to capillary leakage in children with septic shock and purpura. Infect. Immun. 66, 5350–5356 (1998).
Wu, M. C. et al. The receptor for complement component C3a mediates protection from intestinal ischemia-reperfusion injuries by inhibiting neutrophil mobilization. Proc. Natl. Acad. Sci. USA 110, 9439–9444 (2013).
Kovach, M. A. & Standiford, T. J. The function of neutrophils in sepsis. Curr. Opin. Infect. Dis. 25, 321–327 (2012).
Nelson, S. et al. A randomised controlled trial of filgrastim as an adjunct to antibiotics for treatment of hospitalised patients with community-acquired pneumonia. J. Infect. Dis. 178, 1075–1080 (1998).
Rauch, P. J. et al. Innate response activator B cells protect against microbial sepsis. Science 335, 597–601 (2012).
Souza-Fonseca-Guimaraes, F., Cavaillon, J. M. & Adib-Conquy, M. Bench-to-bedside review: natural killer cells in sepsis - guilty or not guilty? Crit. Care 17, 235–240 (2013).
Shao, R. et al. Monocyte programmed death ligand-1 expression after 3-4 days of sepsis is associated with risk stratification and mortality in septic patients: a prospective cohort study. Crit. Care 20, 124–134 (2016).
Drewry, A. M. et al. Comparison of monocyte human leukocyte antigen-DR expression and stimulated tumor necrosis factor alpha production as outcome predictors in severe sepsis: a prospective observational study. Crit. Care 20, 334–344 (2016).
Singer, M. & Deutschman, C. S. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315, 801–810 (2016).
Xu, R. et al. Complement 5a receptor –mediated neutrophil dysfunction is associated with a poor outcome in sepsis. Cell Mol Immunol. 13, 103–109 (2016).
Xu, R. et al. Low expression of CXCR1/2 on neutrophils predicts poor survival in patients with hepatitis B virus-related acutr-on-chronic liver failure. Sci. Rep. 6, 38714–38723 (2016).
Zhang, J. Y. et al. Interleukin-17–producing CD4(+) T cells increase with severity of liver damage in patients with chronic hepatitis B. Hepatology 51, 81–91 (2010).
Acknowledgements
We appreciate the cooperation of all septic individuals, SIRS individuals and healthy volunteers who participated in this study. In addition, we would like to thank the native English-speaking scientists of Elixigen Company (Huntington Beach, California) for editing our manuscript. This work was supported by the National Natural Science Foundation of China [grant number 81400626] and the National Natural Science Foundation for innovation group [grant number 81721002].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval:
This study was reviewed and approved by the Ethics Committee of Beijing 302 Hospital, China.
Informed consent
Written informed consent was obtained from each subject before recruitment.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Huang, H., Wang, S., Jiang, T. et al. High levels of circulating GM-CSF+CD4+ T cells are predictive of poor outcomes in sepsis patients: a prospective cohort study. Cell Mol Immunol 16, 602–610 (2019). https://doi.org/10.1038/s41423-018-0164-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-018-0164-2
Keywords
This article is cited by
-
PKM2/STAT1-mediated PD-L1 upregulation on neutrophils during sepsis promotes neutrophil organ accumulation by serving an anti-apoptotic role
Journal of Inflammation (2023)
-
Granulocyte-macrophage colony-stimulating factor: an immunotarget for sepsis and COVID-19
Cellular & Molecular Immunology (2021)
-
GM-CSF-based treatments in COVID-19: reconciling opposing therapeutic approaches
Nature Reviews Immunology (2020)
-
An immunopathogenic perspective of interleukin-1 signaling
Cellular & Molecular Immunology (2020)