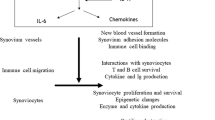
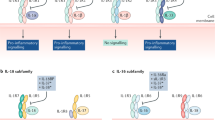
Key Points
-
The IL-23–IL-17 axis is critically involved in the development of autoimmunity
-
Tissue-specific IL-17A expression exacerbates tissue damage and disease chronicity
-
The roles of IL-23 and IL-17 at different stages in the development and progression of rheumatoid arthrits (RA) and spondyloarthritis needs further investigation
-
Greater understanding of the role of the IL-23–IL-17 axis in RA as opposed to psoriatic arthritis and psoriasis is needed
-
Early combination therapy neutralizing both TNF activity and the IL-17/TH17 pathway might be a successful approach to achieving stable remission in patients with autoimmune disease and might even prevent disease development
Abstract
The discovery that the IL-23–IL-17 immune pathway is involved in many models of autoimmune disease has changed the concept of the role of T-helper cell subsets in the development of autoimmunity. In addition to TH17 cells, IL-17 is also produced by other T cell subsets and innate immune cells; which of these IL-17-producing cells have a role in tissue inflammation, and the timing, location and nature of their role(s), is incompletely understood. The current view is that innate and adaptive immune cells expressing the IL-23 receptor become pathogenic after exposure to IL-23, but further investigation into the role of IL-23 and IL-17 at different stages in the development and progression of chronic (destructive) inflammatory diseases is needed. Rheumatoid arthritis (RA) and spondyloarthritis (SpA) are the two most common forms of chronic immune-mediated inflammatory arthritis, and the IL-23–IL-17 axis is thought to have a critical role in both. This Review discusses the basic mechanisms of these cytokines in RA and SpA on the basis of findings from disease-specific animal models as well as human ex vivo studies. Promising therapeutic applications to modulate this immune pathway are in development or have already been approved. Blockade of IL-17 and/or TH17-cell activity in combination with anti-TNF therapy might be a successful approach to achieving stable remission or even prevention of chronic immune-mediated inflammatory diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
15 September 2015
In Figure 2a of this Review, full protection against CIA was incorrectly stated as an effect of IL-17 deficiency instead of IL-17RA deficiency.
References
Lubberts, E. et al. IL-1-independent role of IL-17 in synovial inflammation and joint destruction during collagen-induced arthritis. J. Immunol. 167, 1004–1013 (2001).
Nakae, S., Nambu, A., Sudo, K. & Iwakura, Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J. Immunol. 171, 6173–6177 (2003).
Lubberts, E. et al. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum. 50, 650–659 (2004).
Lubberts, E., Koenders, M. I. & van den Berg, W. B. The role of T-cell interleukin-17 in conducting destructive arthritis: lessons from animal models. Arthritis Res. Ther. 7, 29–37 (2005).
Leipe, J. et al. Role of TH17 cells in human autoimmune arthritis. Arthritis Rheum. 62, 2876–2885 (2010).
Miossec, P. & Kolls, J. K. Targeting IL-17 and TH17 cells in chronic inflammation. Nat. Rev. Drug Discov. 11, 763–776 (2012).
Corneth, O. B. et al. Absence of interleukin-17 receptor A signalling prevents autoimmune inflammation of the joint and leads to a TH2-like phenotype in collagen-induced arthritis. Arthritis Rheum. 66, 340–349 (2014).
Lubberts, E. et al. Overexpression of IL-17 in the knee joint of collagen type II immunized mice promotes collagen arthritis and aggravates joint destruction. Inflamm. Res. 51, 102–104 (2002).
Lubberts, E. et al. Requirement of IL-17 receptor signaling in radiation-resistant cells in the joint for full progression of destructive synovitis. J. Immunol. 175, 3360–3368 (2005).
Koenders, M. I. et al. Interleukin-17 receptor deficiency results in impaired synovial expression of interleukin-1 and matrix metalloproteinases 3, 9, and 13 and prevents cartilage destruction during chronic reactivated streptococcal cell wall-induced arthritis. Arthritis Rheum. 52, 3239–3247 (2005).
Infante-Duarte, C., Horton, H. F., Byrne, M. C. & Kamradt, T. Microbial lipopeptides induce the production of IL-17 in TH cells. J. Immunol. 165, 6107–6115 (2000).
Moseley, T. A., Haudenschild, D. R., Rose, L. & Reddi, A. H. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 14, 155–174 (2003).
Lubberts, E. The role of IL-17 and family members in the pathogenesis of arthritis. Curr. Opin. Investig. Drugs 4, 572–577 (2003).
Kolls, J. K. & Linden, A. Interleukin-17 family members and inflammation. Immunity 21, 467–476 (2004).
Iwakura, Y., Ishigame, H., Saijo, S. & Nakae, S. Functional specialization of interleukin-17 family members. Immunity 34, 149–162 (2011).
Wright, J. F. et al. Identification of an interleukin 17F/17A heterodimer inactivated human CD4+ T cells. J. Biol. Chem. 282, 13447–13455 (2007).
Liang, S. C. et al. An IL-17F/A heterodimer protein is produced by mouse TH17 cells and induces airway neutrophil recruitment. J. Immunol. 179, 7791–7799 (2007).
Toy, D. et al. Cutting Edge: interleukin 17 signals through a heteromeric receptor complex. J. Immunol. 177, 36–39 (2006).
Wright, J. F. et al. The human IL-17F/IL-17A heterodimeric cytokine signals through the IL-17RA/IL-17RC receptor complex. J. Immunol. 181, 2799–2805 (2008).
Gaffen, S. Structure and signalling in the IL-17 receptor family. Nat. Rev. Immunol. 9, 556–567 (2009).
Song, X. et al. IL-17RE is the functional receptor for IL-17C and mediates mucosal immunity to infection with intestinal pathogens. Nat. Immunol. 12, 1151–1158 (2011).
Chang, S. H. et al. Interleukin-17C promotes TH17 cell responses and autoimmune disease via interleukin-17 receptor E. Immunity 35, 611–621 (2011).
Patel, D. D., Lee, D. M., Kolbinger, F. & Antoni, C. Effects of IL-17A blockade with secukinumab in autoimmune diseases. Ann. Rheum. Dis. 72, ii116–ii123 (2013).
Croxford, A. L., Mair, F. & Becher, B. IL-23: one cytokine in control of autoimmunity. Eur. J. Immunol. 42, 2263–2273 (2012).
McGeachy, M. J. et al. TGF-β and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain TH-17 cell-mediated pathology. Nat. Immunol. 8, 1390–1397 (2007).
Zielinski, C. E. et al. Pathogen-induced human TH17 cells produce IFN-γ or IL-10 and are regulated by IL-1β. Nature 484, 514–518 (2012).
Cua, D. J. et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421, 744–748 (2003).
Awasthi, A. et al. Cutting edge: IL-23 receptor GFP reporter mice reveal distinct populations of IL-17-producing cells. J. Immunol. 182, 5904–5908 (2009).
McGeachy, M. J. et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat. Immunol. 10, 314–324 (2009).
Murphy, C. A. et al. Divergent pro- and anti-inflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J. Exp. Med. 198, 1951–1957 (2003).
Oppmann, B. et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13, 715–725 (2000).
Langrish, C. L. et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201, 233–240 (2005).
Harrington, L. E. et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6, 1123–1132 (2005).
Park, H. et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing IL-17. Nat. Immunol. 6, 1133–1141 (2005).
Hueber, A. J. et al. Mast cells express IL-17A in rheumatoid arthritis synovium. J. Immunol. 184, 3336–3340 (2010).
Noordenbos, T. et al. Interleukin-17-positive mast cells contribute to synovial inflammation in spondylarthritis. Arthritis Rheum. 64, 99–109 (2012).
Van Baarsen, L. et al. Heterogeneous expression pattern of interleukin-17A (IL-17A), IL-17F and their receptors in synovium of rheumatoid arthritis, psoriatic arthritis and osteoarthritis: possible explanation for non-response to anti-IL-17 therapy? Arthritis Res. Ther. 16, 426 (2014).
Moran, E. M. et al. IL-17A expression is localised to both mononuclear and polymorphonuclear synovial cell infiltrates. PLoS ONE 6, e24048 (2011).
Appel, H. et al. Analysis of IL-17+ cells in facet joints of patients with spondyloarthritis suggests that the innate immune pathway might be of greater relevance than the TH17-mediated adaptive immune response. Arthritis Res. Ther. 13, R95 (2011).
Cua, D. J. & Tato, C. M. Innate IL-17-producing cells: the sentinels of the immune system. Nat. Rev. Immunol. 10, 479–489 (2010).
Spits, H. et al. Innate lymphoid cells—a proposal for uniform nomenclature. Nat. Immunol. 13, 145–149 (2013).
Cornelissen, F. et al. (2009). Interleukin-23 is critical for full-blown expression of a non-autoimmune destructive arthritis and regulates interleukin-17A and RORγt in γδ T cells. Arthritis Res. Ther. 11, R194 (2009).
El-Behi, M. et al. The encephalitogenicity of TH17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat. Immunol. 12, 568–575 (2011).
Codarri, L. et al. RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat. Immunol. 12, 560–567 (2011).
Campbell, I. K. et al. Protection from collagen-induced arthritis in granulocyte-macrophage colony-stimulating factor-deficient mice. J. Immunol. 161, 3639–3644 (1998).
Plater-Zyberk, C. et al. Combined blockade of granulocyte-macrophage colony stimulating factor and interleukin 17 pathways potently suppresses chronic destructive arthritis in a tumour necrosis factor α-independent mouse model. Ann. Rheum. Dis. 68, 721–728 (2009).
Burmester, G. R. et al. Efficacy and safety of mavrilimumab in subjects with rheumatoid arthritis. Ann. Rheum. Dis. 72, 1445–1452 (2013).
Lubberts, E. TH17 cytokines and arthritis. Semin. Immunopathol. 32, 43–53 (2010).
Korn, T., Bettelli, E., Oukka, M. & Kuchroo, V. K. IL-17 and TH17 cells. Annu. Rev. Immunol. 27, 485–517 (2009).
Qian, Y. et al. Act1, a negative regulator in CD40- and BAFF-mediated B cell survival. Immunity 21, 575–587 (2004).
Pisitkun, P., Claudio, E., Ren, N., Wang, H. & Siebenlist, U. The adapter protein CIKS/ACT1 is necessary for collagen-induced arthritis, and it contributes to the production of collagen-specific antibody. Arthritis Rheum. 62, 3334–3344 (2010).
Luo, Q. et al. A novel disease-modifying antirheumatic drug, iguratimod, ameliorates murine arthritis by blocking IL-17 signaling, distinct from methotrexate and leflunomide. J. Immunol. 191, 4969–4978 (2013).
Hara, M. et al. Safety and efficacy of combination therapy of iguratimod with methotrexate for patients with active rheumatoid arthritis with an inadequate response to methotrexate: an open-label extension of a randomized, double-blind, placebo-controlled trial. Mod. Rheumatol. 24, 410–418 (2014).
Li, J. et al. Efficacy and safety of iguratimod for the treatment of rheumatoid arthritis. Clin. Dev. Immunol. 13, 310628 (2013).
Yago, T. et al. IL-23 induces human osteoclastogenesis via IL-17 in vitro, and anti-IL-23 antibody attentuates collagen-induced arthritis. Arthritis Res. Ther. 9, R96 (2007).
Cornelissen, F. et al. IL-23 dependent and independent stages of experimental arthritis: no clinical effect of therapeutic IL-23p19 inhibition in collagen-induced arthritis. PLoS ONE 8, e57553 (2013).
Krausz, S. et al. Brief report: a phase IIa, randomized, double-blind, placebo-controlled trial of apilimod mesylate, an interleukin-12/interleukin-23 inhibitor, in patients with rheumatoid arthritis. Arthritis Rheum. 64, 1750–1755 (2012).
Adamopoulos, I. E. et al. IL-23 is critical for induction of arthritis, osteoclast formation, and maintenance of bone mass. J. Immunol. 187, 951–959 (2011).
Chen, L., Wei, X. Q., Evans, B., Jiang, W. & Aeschlimann, D. IL23 promotes osteoclast formation by up-regulation of receptor activator of NF-κB (RANK) expression in myeloid precursor cells. Eur. J. Immunol. 38, 2845–2854 (2008).
Li, X. et al. IL-23 induces receptor activator of NF-κB ligand expression in fibroblast-like synoviocytes via STAT3 and NF-κB signal pathways. Imunol. Lett. 127, 100–107 (2010).
Ikeuchi, H. et al. Expression of interleukin-22 in rheumatoid arthritis: potential role as a proinflammatory cytokine. Arthritis Rheum. 52, 1037–1046 (2005).
Kim, K. W. et al. Interleukin-22 promotes osteoclastogenesis in rheumatoid arthritis through induction of RANKL in human synovial fibroblasts. Arthritis Rheum. 64, 1015–1023 (2012).
Geboes, L. et al. Proinflammatory role of the TH17 cytokine interleukin-22 in collagen-induced arthritis in C57BL/6 mice. Arthritis Rheum. 60, 390–395 (2009).
Quinn, J. M. et al. IL-23 inhibits osteoclastogenesis indirectly through lymphocytes and is required for the maintenance of bone mass in mice. J. Immunol. 181, 5720–5729 (2008).
Kamiya, S. et al. Effects of IL-23 and IL-27 on osteoblasts and osteoclasts: inhibitory effects on osteoclast differentiation. J. Bone Mineral Metabol. 25, 277–285 (2007).
Ju, J. H. et al. IL-23 induces receptor activator of NF-κB ligand expression on CD4+ T cells and promotes osteoclastogenesis in an autoimmune arthritis models. J. Immunol. 181, 1507–1518 (2008).
Pöllinger, B. et al. TH17 cells, not IL-17+ γδ T cells, drive arthritic bone destruction in mice and humans. J. Immunol. 186, 2602–2612 (2011).
Van Hamburg, J. P. et al. IL-17/TH17 mediated synovial inflammation is IL-22 independent. Ann. Rheum. Dis. 72, 1700–1707 (2013).
Leipe, J. et al. Interleukin-22 serum levels are associated with radiographic progression in rheumatoid arthritis. Ann. Rheum. Dis. 70, 1453–1457 (2011).
Corneth, O. B. J. et al. Impaired B cell immunity in IL-22 knockout mice in collagen-induced arthritis. Ann. Rheum. Dis. 70 (Suppl. 2), A58–A59 (2011).
Harre, U. et al. Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J. Clin. Invest. 122, 1791–1802 (2012).
Liang, S. C. et al. Interleukin (IL)-22 and IL-17 are coexpressed by TH17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 203, 2271–2279 (2006).
Zheng, Y. et al. Interleukin-22, a TH17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 445, 648–651 (2007).
Mus, A. M. et al. Interleukin-23 promotes TH17 differentiation by inhibiting T-bet and FoxP3 and is required for elevation of interleukin-22, but not IL-21, in autoimmune experimental arthritis. Arthritis Rheum. 62, 1043–1050 (2010).
Firestein, G. S. Evolving concepts of rheumatoid arthritis. Nature 423, 356–361 (2003).
Lubberts, E. IL-17/TH17 targeting: on the road to prevent chronic destructive arthritis? Cytokine 41, 84–91 (2008).
Kotake, S. et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J. Clin. Invest. 103, 1345–1352 (1999).
Chabaud, M. et al. Human interleukin-17: a T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum. 42, 963–970 (1999).
Aarvak, T., Chabaud, M., Kallberg, E., Miossec, P., Natvig, J. B. Change in the TH1/TH2 phenotype of memory T-cell clones from rheumatoid arthritis synovium. Scand. J. Immunol. 50, 1–9 (1999).
Colin, E. M. et al. 1, 25-dihydroxyvitamin D3 modulates TH17 polarization and interleukin-22 expression by memory T cells from patients with early rheumatoid arthritis. Arthritis Rheum. 62, 132–142 (2010).
van Hamburg, J. P. et al. TH17 cells, but not TH1 cells, from patients with early rheumatoid arthritis are potent inducers of matrix metalloproteinases and proinflammatory cytokines upon synovial fibroblast interaction, including autocrine interleukin-17A production. Arthritis Rheum. 63, 73–83 (2011).
Kochi, Y. et al. A regulatory variant in CCR6 is associated with rheumatoid arthritis susceptibility. Nat. Genet. 42, 515–519 (2010).
Yamada, H. et al. TH1 but not TH17 cells predominate in the joints of patients with rheumatoid arthritis. Ann. Rheum. Dis. 67, 1299–1304 (2008).
Zhang, L. et al. Elevated TH22 cells correlated with TH17 cells in patients with rheumatoid arthritis. J. Clin. Immunol. 31, 606–614 (2011).
Nistala, K. et al. Interleukin-17-producing T cells are enriched in the joints of children with arthritis, but have a reciprocal relationship to regulatory T cell numbers. Arthritis Rheum. 58, 875–887 (2008).
Gullick, N. J. et al. Enhanced and persistent levels of IL-17+D4+ T cells and serum IL-17 in patients with early inflammatory arthritis. Clin. Exp. Immunol. 174, 292–301 (2013).
Kim, J. et al. Elevated levels of T helper 17 cells are associated with disease activity in patients with rheumatoid arthritis. Ann. Lab. Med. 33, 52–59 (2013).
Arroyo-Villa, I. et al. Frequency of TH17 CD4+ T cells in early rheumatoid arthritis: a marker of anti-CCP seropositivity. PLoS ONE 7, e42189 (2012).
Miao, J. et al. Frequencies of circulating IL-17-producing CD4+CD161+ T cells and CD4+CD161+ T cells correlate with disease activity in rheumatoid arthritis. Mod. Rheumatol. 24, 265–570 (2014).
Acosta-Rodriguez, E. et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat. Immunol. 8, 639–646 (2007).
Hirota, K. et al. Preferential recruitment of CCR6-expressing TH17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J. Exp. Med. 204, 2803–2812 (2007).
Doodes, P. D. et al. Development of proteoglycan-induced arthritis is independent of IL-17. J. Immunol. 181, 329–337 (2008).
Pöllinger, B. IL-17 producing T cells in mouse models of multiple sclerosis and rheumatoid arthritis. J. Mol. Med. 90, 613–624 (2012).
Koenders, M. I. et al. Blocking of interleukin-17 during reactivation of experimental arthritis prevents joint inflammation and bone erosion by decreasing RANKL and interleukin-1. Am. J. Pathol. 167, 141–149 (2005).
Miossec, P. Interleukin-17 in rheumatoid arthritis. If T cells were to contribute to inflammation and destruction through synergy. Arthritis Rheum. 48, 594–601 (2003).
Bombara, M. P. et al. Cell contact between T cells and synovial fibroblasts causes induction of adhesion molecules and cytokines. J. Leukoc. Biol. 54, 399–406 (1993).
Brennan, F. M. & McInnes, I. B. Evidence that cytokines play a role in rheumatoid arthritis. J. Clin. Invest. 118, 3537–3545 (2008).
McInnes, I. B., Leung, B. P. & Liew, F. Y. Cell–cell interactions in synovitis. Interactions between T lymphocytes and synovial cells. Arthritis Res. 2, 374–378 (2000).
Parsonage, G. et al. Prolonged, granulocyte-macrophage colony-stimulating factor-dependent, neutrophil survival following rheumatoid synovial fibroblast activation by IL-17 and TNFα. Arthritis Res. Ther. 10, R47 (2008).
Tran, C. N. et al. Molecular interactions between T cells and fibroblast-like synoviocytes: role of membrane tumor necrosis factor-α on cytokine-activated T cells. Am. J. Pathol. 171, 1588–1598 (2007).
Ogura, H. et al. Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction. Immunity 29, 628–636 (2008).
Koenders, M. I. et al. Tumor necrosis factor-interleukin-17 interplay induces S100A8, interleukin-1β, and matrix metalloproteinases, and drives irreversible cartilage destruction in murine arthritis: rationale for combination treatment during arthritis. Arthritis Rheum. 63, 2329–2339 (2011).
Zwerina, K. et al. Anti IL-17A therapy inhibits bone loss in TNF-α-mediated murine arthritis by modulation of the T-cell balance. Eur. J. Immunol. 42, 413–423 (2012).
Alzabin, S. et al. Incomplete response of inflammatory arthritis to TNFα blockade is associated with the TH17 pathway. Ann. Rheum. Dis. 71, 1741–1748 (2012).
Eljaafari, A. et al. Bone marrow-derived and synovium-derived mesenchymal cells promote TH17 cell expansion and activation through caspase 1 activation: contribution to the chronicity of rheumatoid arthritis. Arthritis Rheum. 64, 2147–2157 (2012).
Paulissen, S. M. et al. Synovial fibroblasts directly induce TH17 pathogenicity via the cyclooxygenase/prostaglandin E2 pathway, independent of IL-23. J. Immunol. 191, 1364–1372 (2013).
van Hamburg, J. P. et al. TNF blockade requires 1,25(OH)2D3 to control human TH17-mediated synovial inflammation. Ann. Rheum. Dis. 71, 606–612 (2012).
Chen, D. Y. et al. Increasing levels of circulating TH17 cells and interleukin-17 in rheumatoid arthritis patients with an inadequate response to anti-TNF-α therapy. Arthritis Res. Ther. 13, R126 (2011).
Notley, C. A. et al. Blockade of tumor necrosis factor in collagen-induced arthritis reveals a novel immunoregulatory pathway for TH1 and TH17 cells. J. Exp. Med. 205, 2491–2497 (2008).
Aerts, N. E. et al. Increased IL-17 production by peripheral T helper cells after tumour necrosis factor blockade in rheumatoid arthritis is accompanied by inhibition of migration-associated chemokine receptor expression. Rheumatology 49, 2264–2272 (2010).
Kawashiri, S. Y. et al. Proinflammatory cytokines synergistically enhance the production of chemokine ligand 20 (CCL20) from rheumatoid fibroblast-like synovial cells in vitro and serum CCL20 is reduced in vivo by biologic disease-modifying antirheumatic drugs. J. Rheumatol. 36, 2397–2402 (2009).
Evans, H. G. et al. TNF-α blockade induces IL-10 expression in human CD4+ T cells. Nat. Commun. 5, 3199 (2014).
Zielinski, C. E. et al. Pathogen-induced human TH17 cells produce IFN-γ or IL-10 and are regulated by IL-1β. Nature 484, 514–518 (2012).
Genovese, M. C. et al. A phase II randomized study of subcutaneous ixekizumab, an anti-interleukin-17 monoclonal antibody, in rheumatoid arthritis patients who were naive to biologic agents or had an inadequate response to tumor necrosis factor inhibitors. Arthritis Rheum. 66, 1693–1704 (2014).
van den Berg, W. B. & Miossec, P. IL-17 as a future therapeutic target for rheumatoid arthritis. Nat. Rev. Rheumatol. 5, 549–553 (2009).
Pieper, J. et al. CTLA4-Ig (abatacept) therapy modulates T cell effector functions in autoantibody-positive rheumatoid arthritis patients. BMC Immunol. 14, 34 (2013).
Scarsi, M. et al. Reduction of peripheral blood T cells producing IFN-γ and IL-17 after therapy with abatacept for rheumatoid arthritis. Clin. Exp. Rheumatol. 32, 204–210 (2014).
Jain, M. et al. Increased plasma IL-17F levels in rheumatoid arthritis patients are responsive to methotrexate, anti-TNF, and T cell costimulatory modulation. Inflammation 38, 180–186 (2015).
Samson, M. et al. Brief report: inhibition of interleukin-6 function corrects TH17/TREG cell imbalance in patients with rheumatoid arthritis. Arthritis Rheum. 64, 2499–2503 (2012).
Pesce, B. et al. Effect of interleukin-6 receptor blockade on the balance between regulatory T cells and T helper type 17 cells in rheumatoid arthritis patients. Clin. Exp. Immunol. 171, 237–242 (2013).
Hueber, W. et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci. Transl. Med. 2, 52ra72 (2010).
Genovese, M. C. et al. LY2439821, a humanized anti-interleukin-17 monoclonal antibody, in the treatment of patients with rheumatoid arthritis. Arthritis Rheum. 62, 929–939 (2010).
Martin, D. A. et al. A phase 1b multiple ascending dose study evaluating safety, pharmacokinetics, and early clinical response of brodalumab, a human anti-IL-17R antibody, in methotrexate-resistant rheumatoid arthritis. Arthritis Res. Ther. 15, R164 (2013).
Genovese, M. C. et al. Efficacy and safety of secukinumab in patients with rheumatoid arthritis: a phase II, dose-finding, double-blind, randomised, placebo controlled study. Ann. Rheum. Dis. 72, 863–869 (2013).
Genovese, M. C. et al. One-year efficacy and safety results of secukinumab in patients with rheumatoid arthritis: a phase II, dose-finding, double-blind, randomized, placebo-controlled study. J. Rheumatol. 41, 414–421 (2014).
Ye, P. et al. Requirement of interleukin-17 receptor signalling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defence. J. Exp. Med. 194, 519–527 (2001).
Smith, E. et al. IL-17A inhibits the expansion of IL-17A-producing T cells in mice through “short-loop” inhibition via IL-17 receptor. J. Immunol. 181, 1357–1364 (2008).
Mease, P. J. et al. Brodalumab, an anti-IL-17RA monoclonal antibody, in psoriatic arthritis. N. Engl. J. Med. 370, 2295–2306 (2014).
Papp, K. A. et al. Brodalumab, an anti-IL-17-receptor antibody for psoriasis. N. Engl. J. Med. 366, 1181–1189 (2012).
Sherlock, J. P., Buckley, C. D. & Cua, D. J. The critical role of interleukin-23 in spondyloarthropathy. Mol. Immunol. 57, 38–43 (2014).
Hreggvidsdottir, H. S., Noordenbos, T. & Baeten, D. L. Inflammatory pathways in spondyloarthritis. Mol. Immunol. 57, 28–37 (2014).
Noordenbos, T. et al. Interleukin-17-positive mast cells contribute to synovial inflammation in spondylarthritis. Arthritis Rheum. 64, 99–109 (2012).
Lories, R. J. & Baeten, D. L. Differences in pathophysiology between rheumatoid arthritis and ankylosing spondylitis. Clin. Exp. Rheumatol. 27, S10–14 (2009).
Rueda, B. et al. The IL23R Arg381Gln non-synonymous polymorphism confers susceptibility to ankylosing spondylitis. Ann. Rheum. Dis. 67, 1451–1454 (2008).
Reveille, J. D. et al. Genome-wide association study of ankylosing spondylitis identifies non-MHC susceptibility loci. Nat. Genet. 42, 123–127 (2010).
Coffre, M. et al. Combinatorial control of TH17 and TH1 cell function by genetic variation at genes associated with the IL-23 signaling pathway in spondyloarthritis. Arthritis Rheum. 65, 1510–1521 (2013).
Singh, R., Aggarwal, A. & Misra, R. TH1/TH17 cytokine profiles in patients with reactive arthritis/undifferentiated spondyloarthropathy. J. Rheumatol. 34, 2285–2290 (2007).
Jandus, C. et al. Increased numbers of circulating polyfunctional TH17 memory cells in patients with seronegative spondylarthritides. Arthritis Rheum. 58, 2307–2317 (2008).
Shen, H., Goodall, J. C. & Hill Gaston, J. S. Frequency and phenotype of peripheral blood TH17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis Rheum. 60, 1647–1656 (2009).
Zhang, L. et al. Increased frequencies of TH22 cells as well as TH17 cells in the peripheral blood of patients with ankylosing spondylitis and rheumatoid arthritis. PLoS ONE 7, e31000 (2012).
Melis, L. et al. Systemic levels of IL-23 are strongly associated with disease activity in rheumatoid arthritis but not spondyloarthritis. Ann. Rheum. Dis. 69, 618–623 (2010).
Appel, H. et al. Analysis of IL-17+ cells in facet joints of patients with spondyloarthritis suggests that the innate immune pathway might be of greater relevance than the TH17-mediated adaptive immune response. Arthritis Res. Ther. 13, R95 (2011).
Yeremenko, N. & Baeten, D. IL-17 in spondyloarthritis: is the T-party over? Arthritis Res. Ther. 13, 115 (2011).
Kenna, T. J. & Brown, M. A. The role of IL-17-secreting mast cells in inflammatory joint disease. Nat. Rev. Rheum. 9, 375–379 (2013).
Ambarus, C., Yeremenko, N., Tak, P. P. & Baeten, D. Pathogenesis of spondyloarthritis: autoimmune or autoinflammatory? Curr. Opin. Rheumatol. 24, 351–358 (2012).
Ciccia, F. et al. Overexpression of interleukin-23, but not interleukin-17, as an immunologic signature of subclinical intestinal inflammation in ankylosing spondylitis. Arthritis Rheum. 60, 955–965 (2009).
Takahashi, N. et al. IL-17 produced by Paneth cells drives TNF-induced shock. J. Exp. Med. 205, 1755–1761 (2008).
Sherlock, J. P. et al. IL-23 induces spondyloarthropathy by acting on ROR-γt+ CD3+CD4-CD8- entheseal resident T cells. Nat. Med. 18, 1069–1076 (2012).
Ruutu, M. et al. β-glucan triggers spondylarthritis and Crohn's disease-like ileitis in SKG mice. Arthritis Rheum. 64, 2211–2222 (2012).
Benham, H. et al. IL-23-mediates the intestinal response to microbial β-glucan and the development of spondyloarthritis pathology in SKG mice. Arthritis Rheumatol. 66, 1755–1767 (2014).
Bowes, J. et al. Confirmation of TNIP1 and IL23A as susceptibility loci for psoriatic arthritis. Ann. Rheum. Dis. 70, 1641–1644 (2011).
Filer, C. et al. Investigation of association of the IL12B and IL23R genes with psoriatic arthritis. Arthritis Rheum. 58, 3705–3709 (2008).
Hüffmeier, U. et al. Common variants at TRAF3IP2 are associated with susceptibility to psoriatic arthritis and psoriasis. Nat. Genet. 42, 996–999 (2010).
Raychaudhuri, S. P., Raychaudhuri, S. K. & Genovese, M. C. IL-17 receptor and its functional significance in psoriatic arthritis. Mol. Cell. Biochem. 359, 419–429 (2012).
Mrabet, D. et al. Synovial fluid and serum levels of IL-17, IL-23, and CCL-20 in rheumatoid arthritis and psoriatic arthritis: a Tunisian cross-sectional study. Rheumatol. Int. 33, 265–266 (2013).
Tonel, G. et al. Cutting edge: A critical functional role for IL-23 in psoriasis. J. Immunol. 185, 5688–5691 (2010).
Wilson, N. J. et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat. Immunol. 8, 950–957 (2007).
Chan, J. R. et al. IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J. Exp. Med. 203, 2577–2587 (2006).
van der Fits, L. et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 182, 5836–5845 (2009).
Rizzo, H. L. et al. IL-23-mediated psoriasis-like epidermal hyperplasia is dependent on IL-17A. J. Immunol. 186, 1495–1502 (2011).
Cai, Y. et al. Pivotal role of dermal IL-17-producing γδ T cells in skin inflammation. Immunity 35, 596–610 (2011).
Benham, H. et al. TH17 and TH22 cells in psoriatic arthritis and psoriasis. Arthritis Res. Ther. 15, R136 (2013).
Costello, P., Bresnihan, B., O'Farrelly, C. & FitzGerald, O. Predominance of CD8+ T lymphocytes in psoriatic arthritis. J. Rheumatol. 26, 1117 (1999).
Menon, B. et al. Interleukin-17+CD8+ T cells are enriched in the joints of patients with psoriatic arthritis and correlate with disease activity and joint damage progression. Arthritis Rheumatol. 66, 1272–1281 (2014).
Ory, P. A., Gladman, D. D. & Mease, P. J. Psoriatic arthritis and imaging. Ann. Rheum. Dis. 64 (Suppl. 2), ii14–17 (2005).
Novelli, L., Chimenti, M. S., Chiricozzi, A. & Perricone, R. The new era for the treatment of psoriasis and psoriatic arthritis: perspectives and validated strategies. Autoimmun. Rev. 13, 64–69 (2014).
Suzuki, E., Mellins, E. D., Gershwin, M. E., Nestle, F. O. & Adamopoulos, I. E. The IL-23/IL-17 axis in psoriatic arthritis. Autoimmun. Rev. 13, 496–502 (2014).
Gaffen, S. L., Jain, R., Garg, A. V., Cua, D. J. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat. Rev. Immunol. 14, 585–600 (2014).
Leonardi, C. et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N. Engl. J. Med. 366, 1190–1199 (2012).
Rich, P. et al. Secukinumab induction and maintenance therapy in moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled, phase II regimen-finding study. Br. J. Dermatol. 168, 402–411 (2013).
Papp, K. A. et al. Dose-dependent improvement in chronic plaque psoriasis following treatment with anti-IL-23p19 humanized monoclonal antibody (MK-3222) [abstract]. Presented at the American Academy of Dermatology 71st Annual Meeting (2013).
Chiricozzi, A. & Krueger, J. G. IL-17 targeted therapies for psoriasis. Expert Opin. Investig. Drugs 22, 993–1005 (2013).
Kirkham, B. W., Kavanaugh, A. & Reich, K. Interleukin-17A: a unique pathway in immune-mediated diseases: psoriasis, psoriatic arthritis and rheumatoid arthritis. Immunology 141, 133–142 (2014).
Schett, G., Elewaut, D., McInnes, I. B., Dayer, J. M. & Neurath, M. F. How cytokine networks fuel inflammation: toward a cytokine-based disease taxonomy. Nat. Med. 19, 822–824 (2013).
Paulissen, S. M. et al. Higher proportions of pathogenic CCR6+ T cell subpopulations in ACPA+ compared to ACPA− patients with early rheumatoid arthritis. Ann. Rheum. Dis. 73 (Suppl. 1), A33–A34 (2014).
Abbas, A. K., Murphy, K. M., Sher, A. Functional diversity of helper T lymphocytes. Nature 383, 787–793 (1996).
Szabo, S. J. et al. A novel transcription factor, T-bet, directs TH1 lineage commitment. Cell 100, 655–669 (2000).
Szabo, S. J., Sullivan, B. M., Peng, S. L., Glimcher, L. H. Molecular mechanisms regulating TH1 immune responses. Annu. Rev. Immunol. 21, 713–758 (2003).
Kuchroo, V. K. et al. T cell response in experimental autoimmune encephalomyelitis (EAE): role of self and cross-reactive antigens in shaping, tuning, and regulating the autopathogenic T cell repertoire. Annu. Rev. Immunol. 20, 101–123 (2002).
Zheng, W., Flavell, R. A. The transcription factor GATA-3 is necessary and sufficient for TH2 cytokine gene expression in CD4 T cells. Cell 89, 587–596 (1997).
Mosmann, T. R., Coffman, R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7, 145–173 (1989).
Bettelli, E. et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 (2006).
Mangan, P. R. et al. Transforming growth factor-β induces development of the TH17 lineage. Nature 441, 231–234 (2006).
Veldhoen, M. et al. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24, 179–189 (2006).
Ivanov, I. I. et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 (2006).
Bettelli, E., Korn, T., Oukka, M., Kuchroo, V. K. Induction and effector functions of TH17 cells. Nature 453, 1051–1057 (2008).
Kanno, Y., Vahedi, G., Hirahara, K., Singleton, K. & O'Shea, J. J. Transcriptional and epigenetic control of T helper cell specification: molecular mechanisms underlying commitment and plasticity. Annu. Rev. Immunol. 30, 707–731 (2012).
Radhakrishnan, S. et al. Reprogrammed FOXP3+ T regulatory cells become IL-17+ antigen-specific autoimmune effectors in vitro and in vivo. J. Immunol. 181, 3137–3147 (2008).
Xu, L., Kitani, A., Fuss, I. & Strober, W. Cutting edge: regulatory T cells induce CD4+CD25-Foxp3- T cells or are self-induced to become TH17 cells in the absence of exogenous TGF-β. J. Immunol. 178, 6725–6729 (2007).
Yang, X. O. et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR α and ROR γ. Immunity 28, 29–39 (2008).
Koenen, H. J. et al. Human CD25highFoxppos regulatory T cells differentiate into IL-17-producing cells. Blood 112, 2340–2352 (2008).
Komatsu, N. et al. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat. Med. 20, 62–68 (2014).
Lee, Y. K. et al. Late developmental plasticity in the T helper 17 lineage. Immunity 30, 92–107 (2009).
Lexberg, M. H. et al. TH memory for interleukin-17 expression is stable in vivo. Eur. J. Immunol. 38, 2654–2664 (2008).
Martin-Orozco, N., Chung, Y., Chang, S. H., Wang, Y. H. & Dong, C. TH17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into TH1 cells. Eur. J. Immunol. 39, 216–224 (2009).
Wei, G. et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity 30, 155–167 (2009).
Acknowledgements
The author acknowledges team members (past and present) of the Lubberts Lab and the collaborating clinicians and researchers for scientific interactions and helpful discussions regarding this topic, as well as the Dutch Arthritis Association for financial grant support in different projects related to this topic in the past 10 years.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Lubberts, E. The IL-23–IL-17 axis in inflammatory arthritis. Nat Rev Rheumatol 11, 415–429 (2015). https://doi.org/10.1038/nrrheum.2015.53
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2015.53
This article is cited by
-
Application of Cartilage Extracellular Matrix to Enhance Therapeutic Efficacy of Methotrexate
Tissue Engineering and Regenerative Medicine (2024)
-
Evolving cognition of the JAK-STAT signaling pathway: autoimmune disorders and cancer
Signal Transduction and Targeted Therapy (2023)
-
Severe RAS-Associated Lymphoproliferative Disease Case with Increasing αβ Double-Negative T Cells with Atypical Features
Journal of Clinical Immunology (2023)
-
Characterizing memory T helper cells in patients with psoriasis, subclinical, or early psoriatic arthritis using a machine learning algorithm
Arthritis Research & Therapy (2022)
-
Na-AIP-1 secreted by human hookworms suppresses collagen-induced arthritis
Inflammopharmacology (2022)