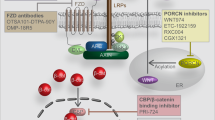
Key Points
-
WNTs are secreted ligands that activate at least two signalling pathways in vertebrates.
-
Altered function or levels of components of the WNT/β-catenin pathway are associated with proliferative diseases including cancer, as well as Alzheimer disease, osteoarthritis, tooth development, and diseases of the bone, eye and heart.
-
Altered function of the WNT/calcium pathway is implicated as a tumour-suppressor pathway, and as a regulator of cancer metastasis.
-
Both activators and inhibitors of WNT pathways are being developed as candidate therapeutic agents.
-
Manipulation of WNT signalling might be important in the development of therapies that are based on human stem cells.
Abstract
WNT signalling has been studied primarily in developing embryos, in which cells respond to WNTs in a context-dependent manner through changes in survival and proliferation, cell fate and movement. But WNTs also have important functions in adults, and aberrant signalling by WNT pathways is linked to a range of diseases, most notably cancer. What is the full range of diseases that involve WNT pathways? Can inhibition of WNT signalling form the basis of an effective therapy for some cancers? Could activation of WNT signalling provide new therapies for other clinical conditions? Finally, on the basis of recent experiments, might WNTs normally participate in self-renewal, proliferation or differentiation of stem cells? If so, altering WNT signalling might be beneficial to the use of stem cells for therapeutic means.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Wodarz, A. & Nusse, R. Mechanisms of Wnt signaling in development. Annu. Rev. Cell Dev. Biol. 14, 59–88 (1998).
Huelsken, J. & Birchmeier, W. New aspects of Wnt signaling pathways in higher vertebrates. Curr. Opin. Genet. Dev. 11, 547–553 (2001).
Veeman, M. T., Axelrod, J. D. & Moon, R. T. A second canon. Functions and mechanisms of β-catenin-independent Wnt signaling. Dev. Cell 5, 367–377 (2003).
Tolwinski, N. S. & Wieschaus, E. Rethinking WNT signaling. Trends Genet. 20, 177–181 (2004).
Nelson, W. J. & Nusse, R. Convergence of Wnt, β-catenin, and cadherin pathways. Science 303, 1483–1487 (2004).
He, X., Semenov, M., Tamai, K. & Zeng, X. LDL receptor-related proteins 5 and 6 in Wnt/β-catenin signaling: arrows point the way. Development 131, 1663–1677 (2004).
Tan, C. et al. Inhibition of integrin linked kinase (ILK) suppresses β-catenin-Lef/Tcf-dependent transcription and expression of the E-cadherin repressor, snail, in APC−/− human colon carcinoma cells. Oncogene 20, 133–140 (2001).
Levina, E., Oren, M. & Ben-Ze'ev, A. Downregulation of β-catenin by p53 involves changes in the rate of β-catenin phosphorylation and Axin dynamics. Oncogene 23, 4444–4453 (2004).
Doble, B. W. & Woodgett, J. R. GSK-3: tricks of the trade for a multi-tasking kinase. J. Cell Sci. 116, 1175–1186 (2003).
Moon, R. T., Bowerman, B., Boutros, M. & Perrimon, N. The promise and perils of Wnt signaling through β-catenin. Science 296, 1644–1646 (2002).
Westfall, T. A. et al. Wnt-5/pipetail functions in vertebrate axis formation as a negative regulator of Wnt/β-catenin activity. J. Cell Biol. 162, 889–898 (2003).
Topol, L. et al. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent β-catenin degradation. J. Cell Biol. 162, 899–908 (2003).
Niemann, S. et al. Homozygous WNT3 mutation causes tetra-amelia in a large consanguineous family. Am. J. Hum. Genet. 74, 558–563 (2004).
Jordan, B. K., Shen, J. H., Olaso, R., Ingraham, H. A. & Vilain, E. Wnt4 overexpression disrupts normal testicular vasculature and inhibits testosterone synthesis by repressing steroidogenic factor 1/β-catenin synergy. Proc. Natl Acad. Sci. USA 100, 10866–10871 (2003).
Perantoni, A. O. Renal development: perspectives on a Wnt-dependent process. Semin. Cell Dev. Biol. 14, 201–208 (2003).
Terada, Y. et al. Expression and function of the developmental gene Wnt-4 during experimental acute renal failure in rats. J. Am. Soc. Nephrol. 14, 1223–1233 (2003).
Surendran, K. & Simon, T. C. CNP gene expression is activated by Wnt signaling and correlates with Wnt4 expression during renal injury. Am. J. Physiol. Renal Physiol. 284, F653–F562 (2003).
Rodova, M., Islam, M. R., Maser, R. L. & Calvet, J. P. The polycystic kidney disease-1 promoter is a target of the β-catenin/T-cell factor pathway. J. Biol. Chem. 277, 29577–29583 (2002).
Olson, D. J. & Gibo, D. M. Antisense wnt-5a mimics wnt-1-mediated C57MG mammary epithelial cell transformation. Exp. Cell Res. 241, 134–141 (1998).
Olson, D. J., Gibo, D. M., Saggers, G., Debinski, W. & Kumar, R. Reversion of uroepithelial cell tumorigenesis by the ectopic expression of human wnt-5a. Cell Growth Differ. 8, 417–423 (1997).
Liang, H. et al. Wnt5a inhibits B cell proliferation and functions as a tumor suppressor in hematopoietic tissue. Cancer Cell 4, 349–360 (2003). Strong evidence that WNT/calcium signalling is a tumour-suppressor pathway.
Weeraratna, A. T. et al. Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell 1, 279–288 (2002). Solid evidence that WNT/calcium signalling is involved in metastasis.
Ouko, L., Ziegler, T. R., Gu, L. H., Eisenberg, L. M. & Yang, V. W. Wnt11 signaling promotes proliferation, transformation and migration of IEC6 intestinal epithelial cells. J. Biol. Chem. 279, 26707–26715 (2004).
Miyaoka, T., Seno, H. & Ishino, H. Increased expression of Wnt-1 in schizophrenic brains. Schizophr. Res. 38, 1–6 (1999).
Katsu, T. et al. The human frizzled-3 (FZD3) gene on chromosome 8p21, a receptor gene for Wnt ligands, is associated with the susceptibility to schizophrenia. Neurosci. Lett. 353, 53–56 (2003).
Kozlovsky, N., Belmaker, R. H. & Agam, G. GSK-3 and the neurodevelopmental hypothesis of schizophrenia. Eur. Neuropsychopharmacol. 12, 13–25 (2002).
Lijam, N. et al. Social interaction and sensorimotor gating abnormalities in mice lacking Dvl1. Cell 90, 895–905 (1997).
Loughlin, J. et al. Functional variants within the secreted frizzled-related protein 3 gene are associated with hip osteoarthritis in females. Proc. Natl Acad. Sci. USA 101, 9757–9762 (2004).
Easwaran, V. et al. β-catenin regulates vascular endothelial growth factor expression in colon cancer. Cancer Res. 63, 3145–3153 (2003).
Robitaille, J. et al. Mutant frizzled-4 disrupts retinal angiogenesis in familial exudative vitreoretinopathy. Nature Genet. 32, 326–330 (2002).
Toomes, C. et al. Mutations in LRP5 or FZD4 underlie the common familial exudative vitreoretinopathy locus on chromosome 11q. Am. J. Hum. Genet. 74, 721–730 (2004).
Kondo, H., Hayashi, H., Oshima, K., Tahira, T. & Hayashi, K. Frizzled 4 gene (FZD4) mutations in patients with familial exudative vitreoretinopathy with variable expressivity. Br. J. Ophthalmol. 87, 1291–1295 (2003).
Xu, Q. et al. Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell 116, 883–895 (2004).
Kaykas, A. et al. Mutant Frizzled 4 associated with vitreoretinopathy traps wild-type Frizzled in the endoplasmic reticulum by oligomerization. Nature Cell Biol. 6, 52–58 (2004).
Harada, S. & Rodan, G. A. Control of osteoblast function and regulation of bone mass. Nature 423, 349–355 (2003).
Johnson, M. L. et al. Linkage of a gene causing high bone mass to human chromosome 11 (11q12-13). Am. J. Hum. Genet. 60, 1326–1332 (1997).
Little, R. D. et al. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am. J. Hum. Genet. 70, 11–19 (2002).
Boyden, L. M. et al. High bone density due to a mutation in LDL-receptor-related protein 5. N. Engl. J. Med. 346, 1513–1521 (2002).
Zhang, Y. et al. The LRP5 high-bone-mass G171V mutation disrupts LRP5 interaction with Mesd. Mol. Cell Biol. 24, 4677–4684 (2004).
Gong, Y. et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell 107, 513–523 (2001). Evidence of LRP5 involvement in bone and eye formation.
Bodine, P. V. et al. The Wnt antagonist secreted frizzled-related protein-1 is a negative regulator of trabecular bone formation in adult mice. Mol. Endocrinol. 18, 1222–1237 (2004).
Kahler, R. A. & Westendorf, J. J. Lymphoid enhancer factor-1 and β-catenin inhibit Runx2-dependent transcriptional activation of the osteocalcin promoter. J. Biol. Chem. 278, 11937–11944 (2003).
Morin, P. J. & Weeraratna, A. T. Wnt signaling in human cancer. Cancer Treat. Res. 115, 169–187 (2003).
Taketo, M. M. Shutting down Wnt signal-activated cancer. Nature Genet. 36, 320–322 (2004).
Giles, R. H., van Es, J. H. & Clevers, H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim. Biophys. Acta 1653, 1–24 (2003).
van de Wetering, M. et al. The β-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell 111, 241–250 (2002).
Uematsu, K. et al. Activation of the Wnt pathway in non small cell lung cancer: evidence of dishevelled overexpression. Oncogene 22, 7218–7221 (2003).
Kim, J. S., Crooks, H., Foxworth, A. & Waldman, T. Proof-of-principle: oncogenic β-catenin is a valid molecular target for the development of pharmacological inhibitors. Mol. Cancer Ther. 1, 1355–1359 (2002).
Gunther, E. J. et al. Impact of p53 loss on reversal and recurrence of conditional Wnt-induced tumorigenesis. Genes Dev. 17, 488–501 (2003).
Derksen, P. W. et al. Illegitimate WNT signaling promotes proliferation of multiple myeloma cells. Proc. Natl Acad. Sci. USA 101, 6122–6127 (2004).
Suzuki, H. et al. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nature Genet. 36, 417–422 (2004).
Kratochwil, K., Galceran, J., Tontsch, S., Roth, W. & Grosschedl, R. FGF4, a direct target of LEF1 and Wnt signaling, can rescue the arrest of tooth organogenesis in Lef1−/− mice. Genes Dev. 16, 3173–3185 (2002).
Lammi, L. et al. Mutations in AXIN2 cause familial tooth agenesis and predispose to colorectal cancer. Am. J. Hum. Genet. 74, 1043–1050 (2004).
Mak, B. C., Takemaru, K., Kenerson, H. L., Moon, R. T. & Yeung, R. S. The tuberin-hamartin complex negatively regulates β-catenin signaling activity. J. Biol. Chem. 278, 5947–5951 (2003).
Fuchs, E., Tumbar, T. & Guasch, G. Socializing with the neighbors: stem cells and their niche. Cell 116, 769–778 (2004).
Chang, C. H. et al. Distinct Wnt members regulate the hierarchical morphogenesis of skin regions (spinal tract) and individual feathers. Mech. Dev. 121, 157–171 (2004).
Zhu, A. J. & Watt, F. M. β-catenin signalling modulates proliferative potential of human epidermal keratinocytes independently of intercellular adhesion. Development 126, 2285–2298 (1999).
Chan, E. F., Gat, U., McNiff, J. M. & Fuchs, E. A common human skin tumour is caused by activating mutations in β-catenin. Nature Genet. 21, 410–413 (1999).
Guo, N., Hawkins, C. & Nathans, J. From the cover: Frizzled6 controls hair patterning in mice. Proc. Natl Acad. Sci. USA 101, 9277–9281 (2004).
Cheon, S. S. et al. β-catenin stabilization dysregulates mesenchymal cell proliferation, motility, and invasiveness and causes aggressive fibromatosis and hyperplastic cutaneous wounds. Proc. Natl Acad. Sci. USA 99, 6973–6978 (2002).
Cheon, S. S., Nadesan, P., Poon, R. & Alman, B. A. Growth factors regulate β-catenin-mediated TCF-dependent transcriptional activation in fibroblasts during the proliferative phase of wound healing. Exp. Cell Res. 293, 267–274 (2004).
Varallo, V. M. et al. β-catenin expression in Dupuytren's disease: potential role for cell-matrix interactions in modulating β-catenin levels in vivo and in vitro. Oncogene 22, 3680–3684 (2003).
Morrisey, E. E. Wnt signaling and pulmonary fibrosis. Am. J. Pathol. 162, 1393–1397 (2003).
Mucenski, M. L. et al. β-catenin is required for specification of proximal/distal cell fate during lung morphogenesis. J. Biol. Chem. 278, 40231–40238 (2003).
Chilosi, M. et al. Aberrant Wnt/β-catenin pathway activation in idiopathic pulmonary fibrosis. Am. J. Pathol. 162, 1495–1502 (2003).
Okubo, T. & Hogan, B. L. Hyperactive Wnt signaling changes the developmental potential of embryonic lung endoderm. J. Biol. 3, 11 (2004).
Hardy, J. & Selkoe, D. J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297, 353–356 (2002).
Terry, A. V. & Buccafusco, J. J. The cholinergic hypothesis of age and Alzheimer's disease-related cognitive deficits: recent challenges and their implications for novel drug development. J. Pharmacol. Exp. Ther. 306, 821–827 (2003).
Zhang, Z. et al. Destabilization of β-catenin by mutations in presenilin-1 potentiates neuronal apoptosis. Nature 395, 698–702 (1998).
Nishimura, M. et al. Presenilin mutations associated with Alzheimer disease cause defective intracellular trafficking of β-catenin, a component of the presenilin protein complex. Nature Med. 5, 164–169 (1999).
Takashima, A. et al. Presenilin 1 associates with glycogen synthase kinase-3β and its substrate tau. Proc. Natl Acad. Sci. USA 95, 9637–9641 (1998).
De Ferrari, G. V. & Inestrosa, N. C. Wnt signaling function in Alzheimer's disease. Brain Res. Brain Res. Rev. 33, 1–12 (2000).
Kang, D. E. et al. Presenilin couples the paired phosphorylation of β-catenin independent of axin: implications for β-catenin activation in tumorigenesis. Cell 110, 751–762 (2002).
Mudher, A. & Lovestone, S. Alzheimer's disease — do tauists and baptists finally shake hands? Trends Neurosci. 25, 22–26 (2002).
Caricasole, A. et al. The Wnt pathway, cell-cycle activation and β-amyloid: novel therapeutic strategies in Alzheimer's disease? Trends Pharmacol. Sci. 24, 233–238 (2003).
Mudher, A. et al. Dishevelled regulates the metabolism of amyloid precursor protein via protein kinase C/mitogen-activated protein kinase and c-Jun terminal kinase. J. Neurosci. 21, 4987–4995 (2001).
Alvarez, G. et al. Lithium protects cultured neurons against β-amyloid-induced neurodegeneration. FEBS Lett. 453, 260–264 (1999).
De Ferrari, G. V. et al. Activation of Wnt signaling rescues neurodegeneration and behavioral impairments induced by β-amyloid fibrils. Mol. Psychiatry 8, 195–208 (2003). Evidence that activating WNT/β-catenin signalling is neuroprotective in vitro and in vivo.
Phiel, C. J., Wilson, C. A., Lee, V. M. & Klein, P. S. GSK-3α regulates production of Alzheimer's disease amyloid-β peptides. Nature 423, 435–439 (2003).
Sun, X. et al. Lithium inhibits amyloid secretion in COS7 cells transfected with amyloid precursor protein C100. Neurosci. Lett. 321, 61–64 (2002).
Olson, E. N. & Schneider, M. D. Sizing up the heart: development redux in disease. Genes Dev. 17, 1937–1956 (2003).
Nakamura, T., Sano, M., Songyang, Z. & Schneider, M. D. A Wnt- and β-catenin-dependent pathway for mammalian cardiac myogenesis. Proc. Natl Acad. Sci. USA 100, 5834–5839 (2003).
Pandur, P., Lasche, M., Eisenberg, L. M. & Kuhl, M. Wnt-11 activation of a non-canonical Wnt signalling pathway is required for cardiogenesis. Nature 418, 636–641 (2002).
van Gijn, M. E., Daemen, M. J., Smits, J. F. & Blankesteijn, W. M. The wnt-frizzled cascade in cardiovascular disease. Cardiovasc. Res. 55, 16–24 (2002).
Barandon, L. et al. Reduction of infarct size and prevention of cardiac rupture in transgenic mice overexpressing FrzA. Circulation 108, 2282–2289 (2003).
Lepourcelet, M. et al. Small-molecule antagonists of the oncogenic Tcf/β-catenin protein complex. Cancer Cell 5, 91–102 (2004).
Emami, K. H. et al. A small molecule inhibitor of β-catenin/CBP transcription. Proc. Natl Acad. Sci. USA (in the press). New lead anti-cancer compound that blocks the WNT/β-catenin pathway.
He, B. et al. A monoclonal antibody against Wnt-1 induces apoptosis in human cancer cells. Neoplasia 6, 7–14 (2004).
You, L. et al. Inhibition of Wnt-2-mediated signaling induces programmed cell death in non-small-cell lung cancer cells. Oncogene 21 June 2004 [epub ahead of print].
Chakrabarty, S., Radjendirane, V., Appelman, H. & Varani, J. Extracellular calcium and calcium sensing receptor function in human colon carcinomas: promotion of E-cadherin expression and suppression of β-catenin/TCF activation. Cancer Res. 63, 67–71 (2003).
Boon, E. M. et al. Sulindac targets nuclear β-catenin accumulation and Wnt signalling in adenomas of patients with familial adenomatous polyposis and in human colorectal cancer cell lines. Br. J. Cancer 90, 224–229 (2004).
Dihlmann, S., Klein, S. & Doeberitz, M. K. Reduction of β-catenin/T-cell transcription factor signaling by aspirin and indomethacin is caused by an increased stabilization of phosphorylated β-catenin. Mol. Cancer Ther. 2, 509–516 (2003).
Nath, N., Kashfi, K., Chen, J. & Rigas, B. Nitric oxide-donating aspirin inhibits β-catenin/T cell factor (TCF) signaling in SW480 colon cancer cells by disrupting the nuclear β-catenin-TCF association. Proc. Natl Acad. Sci. USA 100, 12584–12589 (2003).
Lu, D. et al. Activation of the Wnt signaling pathway in chronic lymphocytic leukemia. Proc. Natl Acad. Sci. USA 101, 3118–3123 (2004).
Thompson, W. J. et al. Exisulind induction of apoptosis involves guanosine 3′,5′-cyclic monophosphate phosphodiesterase inhibition, protein kinase G activation, and attenuated β-catenin. Cancer Res. 60, 3338–3342 (2000).
Zhou, L. et al. Tyrosine kinase inhibitor STI-571/Gleevec downregulates the β-catenin signaling activity. Cancer Lett. 193, 161–170 (2003).
Suksaweang, S. et al. Morphogenesis of chicken liver: identification of localized growth zones and the role of β-catenin/Wnt in size regulation. Dev. Biol. 266, 109–122 (2004).
Chenn, A. & Walsh, C. A. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science 297, 365–369 (2002).
Ross, S. E. et al. Inhibition of adipogenesis by Wnt signaling. Science 289, 950–953 (2000).
Kielman, M. F. et al. Apc modulates embryonic stem-cell differentiation by controlling the dosage of β-catenin signaling. Nature Genet. 32, 594–605 (2002).
Kubo, F., Takeichi, M. & Nakagawa, S. Wnt2b controls retinal cell differentiation at the ciliary marginal zone. Development 130, 587–598 (2003).
Sato, N., Meijer, L., Skaltsounis, L., Greengard, P. & Brivanlou, A. H. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nature Med. 10, 55–63 (2004).
Eaves, C. J. Manipulating hematopoietic stem cell amplification with Wnt. Nature Immunol. 4, 511–512 (2003).
Lee, H. Y. et al. Instructive role of Wnt/β-catenin in sensory fate specification in neural crest stem cells. Science 303, 1020–1023 (2004).
Liu, B. Y., McDermott, S. P., Khwaja, S. S. & Alexander, C. M. The transforming activity of Wnt effectors correlates with their ability to induce the accumulation of mammary progenitor cells. Proc. Natl Acad. Sci. USA 101, 4158–4163 (2004).
Polesskaya, A., Seale, P. & Rudnicki, M. A. Wnt signaling induces the myogenic specification of resident CD45+ adult stem cells during muscle regeneration. Cell 113, 841–852 (2003).
Reya, T. et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature 423, 409–414 (2003).
Willert, K. et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 423, 448–452 (2003). Purification of WNTs and demonstration of their potential effects on stem cells.
Murdoch, B. et al. Wnt-5A augments repopulating capacity and primitive hematopoietic development of human blood stem cells in vivo. Proc. Natl Acad. Sci. USA 100, 3422–3427 (2003).
Castelo-Branco, G. et al. Differential regulation of midbrain dopaminergic neuron development by Wnt-1, Wnt-3a, and Wnt-5a. Proc. Natl Acad. Sci. USA 100, 12747–12752 (2003).
Korinek, V. et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nature Genet. 19, 379–383 (1998).
Kuhnert, F. et al. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc. Natl Acad. Sci. USA 101, 266–271 (2004).
Kim, J. H. et al. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson's disease. Nature 418, 50–56 (2002).
Hori, Y. et al. Growth inhibitors promote differentiation of insulin-producing tissue from embryonic stem cells. Proc. Natl Acad. Sci. USA 99, 16105–16110 (2002).
Acknowledgements
R.T.M. is supported as an investigator and A.K. as an associate of the Howard Hughes Medical Institute, to which we are indebted for support. We thank M. Kahn and P. Yaworsky for reviewing a draft of this manuscript. We acknowledge funding by the Alzheimer's Association and by the National Institutes of Health. G.V.D. is funded by the Pew Latin American Fellows Program in the Biomedical Sciences, and A.D.K. is funded by the National Institutes of Health. As with any review, this one is a snapshot that is blurred by the fast pace of advancement in the field. We therefore apologize for any omissions or oversights.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- PLANAR CELL POLARITY
-
Overt pattern in the plane of the tissue, such as the orientation of hairs or bristles, caused by the subcellular asymmetry in proteins.
- RENAL TUBULES
-
The kidney contains many units known as nephrons, which contain (renal) tubules that are involved in filtering the blood.
- ISCHAEMIA
-
Reduced flow of oxygenated blood to organs in response to blockage in an artery.
- URETER
-
A tube leading from the kidney to the bladder.
- FIBROSIS
-
Formation of fibrous tissue during a repair process.
- siRNA
-
Small interfering RNAs, which target (in a sequence-specific manner) endogenous RNAs for degradation, thereby reducing the function of a gene.
- HAIR MATRIX CELL
-
A cell type at the base of the hair follicle that gives rise to the hair shaft.
- MYOCARDIAL INFARCTION
-
Commonly known as a heart attack, in which there is reduced blood flow to the heart, leading to reduced oxygen supply.
- INTESTINAL CRYPT
-
An involution in the intestine in which stem cells give rise to differentiated cells.
- INTRAPERITONEAL
-
Administered or withdrawn from within the abdominal cavity.
Rights and permissions
About this article
Cite this article
Moon, R., Kohn, A., Ferrari, G. et al. WNT and β-catenin signalling: diseases and therapies. Nat Rev Genet 5, 691–701 (2004). https://doi.org/10.1038/nrg1427
Issue Date:
DOI: https://doi.org/10.1038/nrg1427
This article is cited by
-
PET/CT-based deep learning grading signature to optimize surgical decisions for clinical stage I invasive lung adenocarcinoma and biologic basis under its prediction: a multicenter study
European Journal of Nuclear Medicine and Molecular Imaging (2024)
-
Uncoupling p38α nuclear and cytoplasmic functions and identification of two p38α phosphorylation sites on β-catenin: implications for the Wnt signaling pathway in CRC models
Cell & Bioscience (2023)
-
Effects of advanced glycation end products (AGEs) on the differentiation potential of primary stem cells: a systematic review
Stem Cell Research & Therapy (2023)
-
Oxidative stress-triggered Wnt signaling perturbation characterizes the tipping point of lung adeno-to-squamous transdifferentiation
Signal Transduction and Targeted Therapy (2023)
-
In Vitro Anti-cancer Effect of Crataegus oxyacantha Berry Extract on Hormone Receptor Positive and Triple Negative Breast Cancers via Regulation of Canonical Wnt Signaling Pathway
Applied Biochemistry and Biotechnology (2023)