Key Points
-
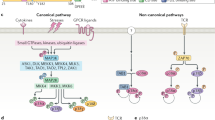
Mitogen-activated protein kinases (MAPKs) integrate and process various extracellular signals. A series of three protein kinases — a MAPK and two upstream components, MAPK kinase (MAPKK) and MAPKK kinase (MAPKKK) — constitutes the MAPK cascade.
-
So far, three distinct MAPK pathways have been described in mammalian cells: the extracellular signal-regulated kinases (ERKs) pathway, the c-Jun amino terminal kinase (JNK) pathway and the p38 MAPK pathway. In general, the ERKs are activated by mitogenic and proliferative stimuli, whereas the JNKs and p38 MAPKs respond to environmental stress, including ultraviolet light, heat, osmotic shock and inflammatory cytokines.
-
Four homologues/isoforms of p38 MAPK have been identified: p38α, p38β, p38γ and p38δ. Of these, p38α is the best characterized and perhaps the most physiologically relevant kinase involved in inflammatory responses.
-
p38 MAPK is activated by dual phosphorylation on Thr180 and Tyr182 by an upstream MAPKK termed MAP2K6. Other MAPKKs, such as MAP2K3, have also been suggested to activate p38 MAPK. MAP2K6 is activated by several MAPKKKs, which are activated by a wide variety of stimuli. An MAPKK-independent mechanism of p38 activation involving TAB1 (transforming growth factor-β-activated protein kinase 1 (TAK1)-binding protein 1) has also been described.
-
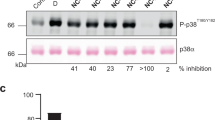
The p38α MAPK pathway is crucial to inflammatory cytokine production and signalling. Several p38 MAPK inhibitors have been shown to block the production of interleukin (IL)-1, tumour-necrosis factor (TNF) and other cytokines. The inhibition of cytokine production seems to result from combined effects at the level of transcription and translation.
-
Several different classes of p38 MAPK inhibitor have been discovered. A large body of evidence from preclinical studies with these inhibitors indicates a crucial role of p38 MAPK in inflammation.
-
On the basis of these studies, several of the more promising compounds have entered human clinical trials for conditions such as rheumatoid arthritis.
Abstract
The p38 MAP kinases are a family of serine/threonine protein kinases that play important roles in cellular responses to external stress signals. Since their identification about 10 years ago, much has been learned of the activation and regulation of the p38 MAP kinase pathways. Inhibitors of two members of the p38 family have been shown to have anti-inflammatory effects in preclinical disease models, primarily through the inhibition of the expression of inflammatory mediators. Several promising compounds have also progressed to clinical trials. In this review, we provide an overview of the role of p38 MAP kinases in stress-activated pathways and the progress towards clinical development of p38 MAP kinase inhibitors in the treatment of inflammatory diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Pearson, G. et al. Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocr. Rev. 22, 153–183 (2001). A comprehensive review of all three MAPK pathways.
Lee, J. C. et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 372, 739–746 (1994). Describes in detail the identification of the molecular target of a class of pyridinyl imidazole compounds known to inhibit cytokine production as p38 MAPK.
Han, J., Lee, J. D., Bibbs, L. & Ulevitch, R. J. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 265, 808–811 (1994). This paper identifies a prominent phosphoprotein extracted from LPS-stimulated mouse macrophage cell line as murine p38 MAPK.
Jiang, Y. et al. Characterization of the structure and function of a new mitogen-activated protein kinase (p38β). J. Biol. Chem. 271, 17920–17926 (1996).
Kumar, S. et al. Novel homologues of CSBP/p38 MAP kinase-activation, substrate specificity and sensitivity to inhibition by pyridinyl imidazoles. Biochem. Biophys. Res. Commun. 235, 533–538 (1997).
Wang, Y. B. et al. Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen-activated protein kinase family. J. Biol. Chem. 273, 2161–2168 (1998). An interesting paper describing the opposing role of various p38 MAPKs in cardiac cells.
Li, Z., Jiang, Y., Ulevitch, R. J. & Han, J. The primary structure of p38γ: a new member of p38 group of MAP kinases. Biochem. Biophys. Res. Commun. 228, 334–340 (1996).
Mertens, S., Craxton, M. & Goedert, M. SAP kinase-3, a new member of the family of mammalian stress-activated protein kinases. FEBS Lett. 383, 273–276 (1996).
Cuenda, A., Cohen, P., Buee-Scherrer, V. & Goedert, M. Activation of stress-activated protein kinase-3 (SAPK3) by cytokines and cellular stresses is mediated via SAPKK3 (MKK6); comparison of the specificities of SAPK3 and SAPK2 (RK/p38). EMBO J. 16, 295–305 (1997).
Court, N. W., dos Remedios, C. G., Cordell, J. & Bogoyevitch, M. A. Cardiac expression and subcellular localization of the p38 mitogen-activated protein kinase member, stress-activated protein kinase-3 (SAPK3). J. Mol. Cell. Cardiol. 34, 413–426 (2002).
Adams, J. L., Badger, A. M., Kumar, S. & Lee, J. C. p38 MAP kinase: molecular target for the inhibition of pro-inflammatory cytokines. Prog. Med. Chem. 38, 1–60 (2001). A comprehensive review of the p38 MAPK pathway, its biology and discovery of various inhibitors.
Kyriakis, J. M. & Avruch, J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 81, 807–869 (2001). A good review of JNK and p38 MAPK pathways.
Ge, B. X. et al. MAPKK-independent activation of p38α mediated by TAB1-dependent autophosphorylation of p38α. Science 295, 1291–1294 (2002). An interesting paper describing TAB1-dependent phosphorylation and activation of p38α MAPK.
Masuda, K., Shima, H., Watanabe, M. & Kikuchi, K. MKP-7, a novel mitogen-activated protein kinase phosphatase, functions as a shuttle protein. J. Biol. Chem. 276, 39002–39011 (2001).
Tanoue, T., Yamamoto, T., Maeda, R. & Nishida, E. A novel MAPK phosphatase MKP-7 acts preferentially on JNK/SAPK and p38α and β MAPKs. J. Biol. Chem. 276, 26629–26639 (2001).
Theodosiou, A., Smith, A., Gillieron, C., Arkinstall, S. & Ashworth, A. MKP5, a new member of the MAP kinase phosphatase family, which selectively dephosphorylates stress-activated kinases. Oncogene 18, 6981–6988 (1999).
Allen, M. et al. Deficiency of the stress kinase p38α results in embryonic lethality: Characterization of the kinase dependence of stress response of enzyme-deficient embryonic stem cells. J. Exp. Med. 191, 859–869 (2000). This paper describes the phenotype of embryonic cells derived from p38-null mice.
Kotlyarov, A. et al. MAPKAP kinase 2 is essential for LPS-induced TNF-α biosynthesis. Nature Cell Biol. 1, 94–97 (1999).
Caput, D. et al. Identification of a common nucleotide sequence in the 3'-untranslated region of mRNA molecules specifying inflammatory mediators. Proc. Natl Acad. Sci. USA 83, 1670–1674 (1986).
Shaw, G. & Kamen, R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell 46, 659–667 (1986).
Winzen, R. et al. The p38 MAP kinase pathway signals for cytokine-induced mRNA stabilization via MAP kinase-activated protein kinase 2 and an AU-rich region-targeted mechanism. EMBO J. 18, 4969–4980 (1999). This paper, together with references 22–24, provides evidence for a role of the p38 MAPK pathway in stabilization of short-lived mRNAs.
Ming, X. F., Stoecklin, G., Lu, M., Looser, R. & Moroni, C. Parallel and independent regulation of interleukin-3 mRNA turnover by phosphatidylinositol 3-kinase and p38 mitogen-activated protein kinase. Mol. Cell. Biol. 21, 5778–5789 (2001).
Neininger, A. et al. MK2 targets AU-rich elements and regulates biosynthesis of tumor necrosis factor and interleukin-6 independently at different post-transcriptional levels. J. Biol. Chem. 277, 3065–3068 (2002).
Frevel, M. A. E. et al. p38 mitogen-activated protein kinase-dependent and -independent signaling of mRNA stability of AU-rich element-containing transcripts. Mol. Cell. Biol. 23, 425–436 (2003).
Lantos, I. et al. Antiinflammatory activity of 5,6-diaryl-2,3-dihydroimidazo[2,1-b]thiazoles. Isomeric 4-pyridyl and 4-substituted phenyl derivatives. J. Med. Chem. 27, 72–75 (1984).
Lee, J. C., Griswold, D. E., Votta, B. & Hanna, N. Inhibition of monocyte IL-1 production by the anti-inflammatory compound, SK&F 86002. Int. J. Immunopharmacol. 10, 835–843 (1988).
Lee, J. C. et al. Bicyclic imidazoles as a novel class of cytokine biosynthesis inhibitors. Ann. NY Acad. Sci. 696, 149–170 (1993).
Lee, J. C., Kassis, S., Kumar, S., Badger, A. & Adams, J. L. p38 mitogen-activated protein kinase inhibitors. Mechanism and therapeutic potentials. Pharmacol. Ther. 82, 389–397 (1999).
Gallagher, T. F. et al. 2,4,5-Triarylimidazole inhibitors of IL-1 biosynthesis. Bioorg. Med. Chem. Lett. 5, 1171–1176 (1995).
Gallagher, T. et al. Regulation of stress-induced cytokine production by pyridinylimidazoles; inhibition of CSBP kinase. Bioorg. Med. Chem. 5, 49–64 (1997).
Adams, J. L. et al. Pyrimidinylimidazole inhibitors of CSBP/p38 kinase demonstrating decreased inhibition of hepatic cytochrome P450 enzymes. Bioorg. Med. Chem. Lett. 8, 3111–3116 (1998).
Boehm, J. C. et al. 1-substituted 4-aryl-5-pyridinylimidazoles: a new class of cytokine suppressive drugs with low 5-lipoxygenase and cyclooxygenase inhibitory potency. J. Med. Chem. 39, 3929–3937 (1996).
Adams, J. L. et al. Pyrimidinylimidazole inhibitors of p38: Cyclic N-1 imidazole substituents enhance p38 kinase inhibition and oral activity. Bioorg. Med. Chem. Lett. 11, 2867–2870 (2001).
Liverton, N. J. et al. Design and synthesis of potent, selective, and orally bioavailable tetrasubstituted imidazole inhibitors of p38 mitogen-activated protein kinase. J. Med. Chem. 42, 2180–2190 (1999).
Boehm, J. C. & Adams, J. L. New inhibitors of p38 kinase. Expert Opin. Ther. Patents 10, 25–37 (2000). This review of the p38 MAPK inhibitor patent literature through 1999 includes a summary of the SAR of the pyridinyl imidazole-based analogues. The molecular basis of the SAR is also highlighted.
Jackson, P. F. & Bullington, J. L. Pyridinylimidazole based p38 MAP kinase inhibitors. Curr. Top. Med. Chem. 2, 1011–1020 (2002).
Cirillo, P. F., Pargellis, C. & Regan, J. The non-diaryl heterocycle classes of p38 MAP kinase inhibitors. Curr. Top. Med. Chem. 2, 1021–1035 (2002). This is a thorough review of potent p38 MAPK inhibitors unrelated to pyridinyl imidazole-based p38 MAPK inhibitors.
Wilson, K. P. et al. Crystal structure of p38 mitogen-activated protein kinase. J. Biol. Chem. 271, 27696–27700 (1996). First published report on the crystal structure of the p38 MAPK apoenzyme.
Wang, Z. et al. The structure of mitogen-activated protein kinase p38 at 2.1 Å resolution. Proc. Natl Acad. Sci. USA 94, 2327–2332 (1997).
Wang, Z. L. et al. Structural basis of inhibitor selectivity in MAP kinases. Structure 6, 1117–1128 (1998).
Wilson, K. P. et al. The structural basis for the specificity of pyridinylimidazole inhibitors of p38 MAP kinase. Chem. Biol. 4, 423–431 (1997).
Tong, L. et al. A highly specific inhibitor of human p38 MAP kinase binds in the ATP pocket. Nature Struct. Biol. 4, 311–316 (1997). First report of a co-crystal structure of an inhibitor bound to the p38 MAPK.
Gum, R. J. et al. Acquisition of sensitivity of stress-activated protein kinases to the p38 inhibitor, SB 203580, by alteration of one or more amino acids within the ATP binding pocket. J. Biol. Chem. 273, 15605–15610 (1998). A comprehensive analysis of various amino acid substitutions at Thr106 of p38 MAPK and sensitivity to SB-203580.
Fox, T. et al. A single amino acid substitution makes ERK2 susceptible to pyridinyl imidazole inhibitors of p38 MAP kinase. Protein Sci. 7, 2249–2255 (1998).
Eyers, P. A., Craxton, M., Morrice, N., Cohen, P. & Goedert, M. Conversion of SB 203580-insensitive MAP kinase family members to drug-sensitive forms by a single amino-acid substitution. Chem. Biol. 5, 321–328 (1998). Describes the importance of the Thr106 residue in defining the space requirement for binding by the diaryl imidazole inhibitors of p38 MAPK.
Pargellis, C. et al. Inhibition of p38 MAP kinase by utilizing a novel allosteric binding site. Nature Struct. Biol. 9, 268–272 (2002).
Regan, J. et al. Pyrazole urea-based inhibitors of p38 MAP kinase: From lead compound to clinical candidate. J. Med. Chem. 45, 2994–3008 (2002).
Badger, A. M. et al. Differential effects of SB 242235, a selective p38 mitogen-activated protein kinase inhibitor, on IL-1 treated bovine and human cartilage/chondrocyte cultures. Osteoarthr. Cartil. 8, 434–443 (2000).
Guan, Z. H., Buckman, S. Y., Pentland, A. P., Templeton, D. J. & Morrison, A. R. Induction of cyclooxygenase-2 by the activated MEKK1-/SEK1/MKK4-/p38 mitogen-activated protein kinase pathway. J. Biol. Chem. 273, 12901–12908 (1998).
Saccani, S., Pantano, S. & Natoli, G. p38-dependent marking of inflammatory genes for increased NF-κB recruitment. Nature Immunol. 3, 69–75 (2002). An interesting paper describing a p38 MAPK-dependent increase in expression of inflammatory cytokines and chemokines.
Bendele, A. M. et al. Combination benefit of treatment with the cytokine inhibitors interleukin-1 receptor antagonist and PEGylated soluble tumor necrosis factor receptor type I in animal models of rheumatoid arthritis. Arthritis Rheum. 43, 2648–2659 (2000).
Kalden, J. R. How do the biologics fit into the current DMARD armamentarium? J. Rheumatol. Suppl. 62, 27–35 (2001).
Smolen, J. S. & Steiner, G. Therapeutic strategies for rheumatoid arthritis. Nature Rev. Drug Discov. 2, 473–488 (2003). A recent comprehensive review on rheumatoid arthritis.
Badger, A. M. et al. Pharmacological profile of SB 203580, a selective inhibitor of cytokine suppressive binding protein/p38 kinase, in animal models of arthritis, bone resorption, endotoxin shock and immune function. J. Pharmacol. Exp. Therap. 279, 1453–1461 (1996).
Ma, X. L. et al. Inhibition of p38 mitogen-activated protein kinase decreases cardiomyocyte apoptosis and improves cardiac function after myocardial ischemia and reperfusion. Circulation 99, 1685–1691 (1999).
Wadsworth, S. A. et al. RWJ 67657, a potent, orally active inhibitor of p38 mitogen-activated protein kinase. J. Pharmacol. Exp. Ther. 291, 680–687 (1999).
Badger, A. M. et al. Disease-modifying activity of SB 242235, a selective inhibitor of p38 mitogen-activated protein kinase, in rat adjuvant-induced arthritis. Arthritis Rheum. 43, 175–183 (2000). Describes the efficacy of a p38 MAPK inhibitor in a rat in vivo model of rheumatoid arthritis.
Revesz, L. et al. SAR of 4-hydroxypiperidine and hydroxyalkyl substituted heterocycles as novel p38 MAP kinase inhibitors. Bioorg. Med. Chem. Lett. 10, 1261–1264 (2000).
McLay, I. M. et al. The discovery of RPR 200765A, a p38 MAP kinase inhibitor displaying a good oral anti-arthritic efficacy. Bioorg. Med. Chem. 9, 537–554 (2001).
McKenna, J. M. et al. An algorithm-directed two-component library synthesized via solid-phase methodology yielding potent and orally bioavailable p38 MAP kinase inhibitors. J. Med. Chem. 45, 2173–2184 (2002).
Behr, T. M. et al. Hypertensive end-organ damage and premature mortality are p38 mitogen-activated protein kinase-dependent in a rat model of cardiac hypertrophy and dysfunction. Circulation 104, 1292–1298 (2001). Describes the role of p38 MAPK in an in vivo model of cardiac hypertrophy.
Hull, M., Lieb, K. & Fiebich, B. L. Pathways of inflammatory activation in Alzheimer's disease: Potential targets for disease modifying drugs. Curr. Med. Chem. 9, 83–88 (2002).
Kawashima, Y. et al. FR167653 attenuates ischemia and reperfusion injury of the rat lung with suppressing p38 mitogen-activated protein kinase. J. Heart Lung Transplant. 20, 568–574 (2001).
Kobayashi, M., Takeyoshi, I., Yoshinari, D., Matsumoto, K. & Morishita, Y. P38 mitogen-activated protein kinase inhibition attenuates ischemia-reperfusion injury of the rat liver. Surgery 131, 344–349 (2002). Describes the role of p38 MAPK in ischaemia and reperfusion.
Ju, H. S. et al. Sustained activation of p38 mitogen-activated protein kinase contributes to the vascular response to injury. J. Pharmacol. Exp. Ther. 301, 15–20 (2002).
Waetzig, G. H., Seegert, D., Rosenstiel, P., Nikolaus, S. & Schreiber, S. p38 mitogen-activated protein kinase is activated and linked to TNF-α signaling in inflammatory bowel disease. J. Immunol. 168, 5342–5351 (2002).
Fullerton, T. et al. Suppression of ex vivo cytokine production by SB-242235, a selective inhibitor of p38 MAP kinase. 101st Ann. Meet. Am. Soc. Clin. Pharmacol. 67, 114 (2000).
Fijen, J. W. et al. Suppression of the clinical and cytokine response to endotoxin by RWJ-67657, a p38 mitogen-activated protein-kinase inhibitor, in healthy human volunteers. Clin. Exp. Immunol. 124, 16–20 (2001). Describes the inhibition of inflammatory cytokines by a p38 MAPK inhibitor in healthy human volunteers.
Weisman, M. et al. Double-blind, placebo-controlled trial of VX-745, an oral p38 mitogen activated protein kinase inhibitor, in patients with rheumatoid arthritis (RA). Ann. European Congress Rheumatol. A10018 (2002).
Wood, C. C., Yong, C. L., Madwed, J. B. & Gupta, A. Pharmacokinetics and pharmacodynamics of an oral p38 MAP kinase inhibitor (BIRB 796 BS), administered once daily for 7 days. 58th Ann. Meet. Am. Acad. Allergy, Asthma Immunol. 109, S321–A993 (2002).
Gupta, A. et al. Safety, pharmacokinetics, and pharmacodynamics of single doses of an oral p38 MAP kinase inhibitor (BIRB 796 BS) in healthy human males, a placebo controlled, randomized study, double blinded at each dose level. Ann. Meet. Am. Acad. Allergy, Asthma Immunol. 109, S67–A158 (2002).
Polmar, S. H. et al. Safety and pharmacokinetics of an oral p38 MAP kinase inhibitor (BIRB 796 BS), administered twice daily for 14 days to healthy volunteers. 58th Ann. Meet. Am. Acad. Allergy, Asthma Immunol. 109, S66–A167 (2002).
Goldstein, D. M. The discovery and development of selective inhibitors of p38 MAP kinase from distinct chemical classes. J. Inflamm. Res. 51, S114 (2002).
Fearns, C. et al. Coordinate activation of endogenous p38 α, β, γ, and δ by inflammatory stimuli. J. Leukoc. Biol. 67, 705–711 (2000).
Barone, F. C. et al. SB 239063, a second-generation p38 mitogen-activated protein kinase inhibitor, reduces brain injury and neurological deficits in cerebral focal ischemia. J. Pharmacol. Exp. Ther. 296, 312–321 (2001).
Acknowledgements
The authors wish to acknowledge N. Nevins for her contribution to the illustration in Fig. 5 and S. Blake for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
LocusLink
Online Mendelian Inheritance in Man
FURTHER INFORMATION
Encyclopedia of Life Sciences
Glossary
- 3′ UNTRANSLATED REGION
-
Part of the sequence in a messenger RNA molecule at the 3′ end of the coding sequence that is not translated. This structural feature is involved in mRNA stability or turnover.
- PHARMACOPHORE
-
The ensemble of steric and electronic features that is necessary to ensure optimal interactions with a specific biological target structure and to trigger (or to block) its biological response.
- CRYPTIC PROMOTERS
-
Promoter regions that do not contain recognized sequence elements corresponding to a classical promoter sequence.
- ACR20
-
A 20% response rate according to American College of Rheumatology clinical classification criteria.
Rights and permissions
About this article
Cite this article
Kumar, S., Boehm, J. & Lee, J. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nat Rev Drug Discov 2, 717–726 (2003). https://doi.org/10.1038/nrd1177
Issue Date:
DOI: https://doi.org/10.1038/nrd1177
This article is cited by
-
Increased Expression of KNa1.2 Channel by MAPK Pathway Regulates Neuronal Activity Following Traumatic Brain Injury
Neurochemical Research (2024)
-
Bacterial extracellular vesicles repress the vascular protective factor RNase1 in human lung endothelial cells
Cell Communication and Signaling (2023)
-
CNS-Active p38α MAPK Inhibitors for the Management of Neuroinflammatory Diseases: Medicinal Chemical Properties and Therapeutic Capabilities
Molecular Neurobiology (2023)
-
Inhibition of importin-7 attenuates ventilator-induced lung injury by targeting nuclear translocation of p38
Inflammation Research (2023)
-
Phenotype-based screening rediscovered benzopyran-embedded microtubule inhibitors as anti-neuroinflammatory agents by modulating the tubulin–p65 interaction
Experimental & Molecular Medicine (2022)