Abstract
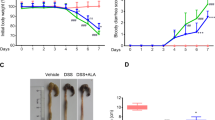
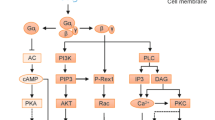
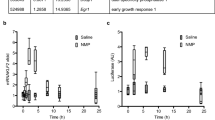
Aspirin (ASA) and dexamethasone (DEX) are widely used anti-inflammatory agents yet their mechanism(s) for blocking polymorphonuclear neutrophil (PMN) accumulation at sites of inflammation remains unclear. Here, we report that inhibition of PMN infiltration by ASA and DEX is a property shared by aspirin-triggered lipoxins (ATL) and the glucocorticoid-induced annexin 1 (ANXA1)-derived peptides that are both generated in vivo and act at the lipoxin A4 receptor (ALXR/FPRL1) to halt PMN diapedesis. These structurally diverse ligands specifically interact directly with recombinant human ALXR demonstrated by specific radioligand binding and function as well as immunoprecipitation of PMN receptors. In addition, the combination of both ATL and ANXA1-derived peptides limited PMN infiltration and reduced production of inflammatory mediators (that is, prostaglandins and chemokines) in vivo. Together, these results indicate functional redundancies in endogenous lipid and peptide anti-inflammatory circuits that are spatially and temporally separate, where both ATL and specific ANXA1-derived peptides act in concert at ALXR to downregulate PMN recruitment to inflammatory loci.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Cotran, R.S. Inflammation: Historical perspectives. in Inflammation: Basic Principles and Clinical Correlates 3rd edn. (eds. Gallin, J.I., Snyderman, R., Fearon, D.T., Haynes, B.F. & Nathan, C.) 5–10 (Lippincott Williams & Wilkins, Philadelphia, 1999).
Weissmann, G. Aspirin. Sci. Am. 264, 84–90 (1991).
Patrono, C. Aspirin: new cardiovascular uses for an old drug. Am. J. Med. 110, 62S–65S (2001).
Barnes, C.J., Hardman, W.E., Cameron, I.L. & Lee, M. Aspirin, but not sodium salicylate, indomethacin, or nabumetone, reversibly suppresses 1,2-dimethylhydrazine-induced colonic aberrant crypt foci in rats. Dig. Dis. Sci. 42, 920–926 (1997).
Marcus, A.J. Aspirin as prophylaxis against colorectal cancer. N. Engl. J. Med. 333, 656–658 (1995).
Samuelsson, B. From studies of biochemical mechanisms to novel biological mediators: Prostaglandin endoperoxides, thromboxanes and leukotrienes. in Les Prix Nobel: Nobel Prizes, Presentations, Biographies and Lectures 153–174 (Almqvist & Wiksell, Stockholm, 1982).
Vane, J.R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nature (London) New Biol. 231, 232–235 (1971).
Hench, P.S., Kendall, E.C., Slocumb, C.H. & Polley, H.E. Proc. Staff Meet. Mayo Clin. 24, 181 (1949).
Boumpas, D.T. & Wilder, R.L. Corticosteroids. in Arthritis and Allied Conditions: A Textbook of Rheumatology 14th edn. edn (ed. Koopman, W.J.) 827–847 (Lippincott Williams & Wilkins, Philadelphia, 2001).
Derry, S. & Loke, Y.K. Risk of gastrointestinal haemorrhage with long term use of aspirin: meta-analysis. Br. Med. J. 321, 1183–1187 (2000).
Cronstein, B.N. & Weissmann, G. Targets for antiinflammatory drugs. Annu. Rev. Pharmacol. Toxicol. 35, 449–462 (1995).
Sin, Y.M., Sedgwick, A.D., Chea, E.P. & Willoughby, D.A. Mast cells in newly formed lining tissue during acute inflammation: A six day air pouch model in the mouse. Ann. Rheum. Dis. 45, 873–877 (1986).
Jick, H., Pinals, R.S., Ullian, R., Slone, D. & Muench, H. Dexamethasone and dexamethasone-aspirin in the treatment of chronic rheumatoid arthritis. Lancet 2, 1203–1205 (1965).
Flower, R.J. & Rothwell, N.J. Lipocortin-1: Cellular mechanisms and clinical relevance. Trends Pharmacol. Sci. 15, 71–76 (1994).
Lim, L.H., Solito, E., Russo-Marie, F., Flower, R.J. & Perretti, M. Promoting detachment of neutrophils adherent to murine postcapillary venules to control inflammation: effect of lipocortin 1. Proc. Natl. Acad. Sci USA 95, 14535–14539 (1998).
Maridonneau-Parini, I., Errasfa, M. & Russo-Marie, F. Inhibition of O2-generation by dexamethasone is mimicked by lipocortin I in alveolar macrophages. J. Clin. Invest. 83, 1936–1940 (1989).
Serhan, C.N., Takano, T., Chiang, N., Gronert, K. & Clish, C.B. Formation of endogenous “antiinflammatory” lipid mediators by transcellular biosynthesis: Lipoxins and aspirin-triggered lipoxins inhibit neutrophil recruitment and vascular permeability. Am. J. Respir. Crit. Care Med. 161, S95–S101 (2000).
Chiang, N., Fierro, I.M., Gronert, K. & Serhan, C.N. Activation of lipoxin A4 receptors by aspirin-triggered lipoxins and select peptides evokes ligand-specific responses in inflammation. J. Exp. Med. 191, 1197–1207 (2000).
Yokomizo, T., Izumi, T., Chang, K., Takuwa, T. & Shimizu, T. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature 387, 620–624 (1997).
Gabay, C. & Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 340, 448–454 (1999).
Su, S.B. et al. A seven-transmembrane, G protein-coupled receptor, FPRL1, mediates the chemotactic activity of serum amyloid A for human phagocytic cells. J. Exp. Med. 189, 395–402 (1999).
Linke, R.P., Bock, V., Valet, G. & Rothe, G. Inhibition of the oxidative burst response of N-formyl peptide-stimulated neutrophils by serum amyloid-A protein. Biochem. Biophys. Res. Commun. 176, 1100–1105 (1991).
Oliani, S.M., Paul-Clark, M.J., Christian, H.C., Flower, R.J. & Perretti, M. Neutrophil interaction with inflamed postcapillary venule endothelium alters annexin 1 expression. Am. J. Pathol. 158, 603–615 (2001).
Perretti, M. et al. Mobilizing lipocortin 1 in adherent human leukocytes downregulates their transmigration. Nature Med. 2, 1259–1262 (1996).
Walther, A., Riehemann, K. & Gerke, V. A novel ligand of the formyl peptide receptor: annexin I regulates neutrophil extravasation by interacting with the FPR. Mol. Cell. 5, 831–840 (2000).
Perretti, M., Getting, S.J., Solito, E., Murphy, P.M. & Gao, J.L. Involvement of the receptor for formylated peptides in the in vivo anti-migratory actions of annexin 1 and its mimetics. Am. J. Pathol. 158, 1969–1973 (2001).
Papayianni, A., Serhan, C.N. & Brady, H.R. Lipoxin A4 and B4 inhibit leukotriene-stimulated interactions of human neutrophils and endothelial cells. J. Immunol. 156, 2264–2272 (1996).
Kang, Y., Taddeo, B., Varai, G., Varga, J. & Fiore, S. Mutations of serine 236–237 and tyrosine 302 residues in the human lipoxin A4 receptor intracellular domains result in sustained signaling. Biochemistry 39, 13551–13557 (2000).
Chiang, N. et al. Aspirin-triggered 15-epi-lipoxin A4 (ATL) generation by human leukocytes and murine peritonitis exudates: Development of a specific 15-epi-LXA4 ELISA. J. Pharmacol. Exp. Ther. 287, 779–790 (1998).
Clish, C.B. et al. Local and systemic delivery of a stable aspirin-triggered lipoxin prevents neutrophil recruitment in vivo. Proc. Natl. Acad. Sci. USA 96, 8247–8252 (1999).
Fadok, V.A. et al. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature 405, 28–29 (2000).
Godson, C. et al. Cutting edge: Lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J. Immunol. 164, 1663–1667 (2000).
Liu, Y. et al. Glucocorticoids promote nonphlogistic phagocytosis of apoptotic leukocytes. J. Immunol. 162, 3639–3646 (1999).
Tsao, F.H.C., Meyer, K.C., Chen, X., Rosenthal, N.S. & Hu, J. Degradation of annexin I in bronchoalveolar lavage fluid from patients with cystic fibrosis. Am. J. Respir. Cell Mol. Biol. 18, 120–128 (1998).
Ariel, A. et al. IL-2 induces T cell adherence to extracellular matrix: Inhibition of adherence and migration by IL-2 peptides generated by leukocyte elastase. J. Immunol. 161, 2465–2472 (1998).
Yokomizo, T., Izumi, T. & Shimizu, T. Leukotriene B4: Metabolism and signal transduction. Arch. Biochem. Biophys. 385, 231–241 (2001).
Perretti, M. et al. Acute inflammatory response in the mouse: Exacerbation by immunoneutralization of lipocortin 1. Br. J. Pharmacol. 117, 1145–1154 (1996).
Cotter, M.J., Norman, K.E., Hellewell, P.G. & Ridger, V.C. A novel method for isolation of neutrophils from murine blood using negative immunomagnetic separation. Am. J. Pathol. 159, 473–481 (2001).
Acknowledgements
We thank B. Schmidt for microscopic analyses, N. Petasis for synthetic LXA4 and ATL stable analogs (prepared for P01-DE13499), and M.H. Small for expert assistance in manuscript preparation. This work was supported in part by grants GM38765 and P01-DE13499 (to C.N.S.) and by grants P0567 and P0583 of the Arthritis Research Campaign UK (to M.P.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Perretti, M., Chiang, N., La, M. et al. Endogenous lipid- and peptide-derived anti-inflammatory pathways generated with glucocorticoid and aspirin treatment activate the lipoxin A4 receptor. Nat Med 8, 1296–1302 (2002). https://doi.org/10.1038/nm786
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm786
This article is cited by
-
Development of an Inflamed High Throughput Stem-cell-based Gut Epithelium Model to Assess the Impact of Annexin A1
Stem Cell Reviews and Reports (2024)
-
Formyl peptide receptor 2 is an emerging modulator of inflammation in the liver
Experimental & Molecular Medicine (2023)
-
Formyl peptide receptor 2, as an important target for ligands triggering the inflammatory response regulation: a link to brain pathology
Pharmacological Reports (2021)
-
The formyl peptide receptor agonist Ac2-26 alleviates neuroinflammation in a mouse model of pneumococcal meningitis
Journal of Neuroinflammation (2020)
-
Application of small molecule FPR1 antagonists in the treatment of cancers
Scientific Reports (2020)