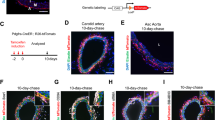
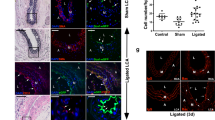
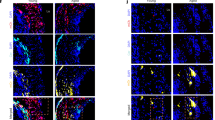
Abstract
Excessive accumulation of smooth-muscle cells (SMCs) has a key role in the pathogenesis of vascular diseases. It has been assumed that SMCs derived from the outer medial layer migrate, proliferate and synthesize extracellular matrix components on the luminal side of the vessel. Although much effort has been devoted to targeting migration and proliferation of medial SMCs, there is no effective therapy that prevents occlusive vascular remodeling. We show here that in models of post-angioplasty restenosis, graft vasculopathy and hyperlipidemia-induced atherosclerosis, bone-marrow cells give rise to most of the SMCs that contribute to arterial remodeling. Notably, purified hematopoietic stem cells differentiate into SMCs in vitro and in vivo. Our findings indicate that somatic stem cells contribute to pathological remodeling of remote organs, and may provide the basis for the development of new therapeutic strategies for vascular diseases through targeting mobilization, homing, differentiation and proliferation of bone marrow-derived vascular progenitor cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ross, R. & Glomset, J.A. Atherosclerosis and the arterial smooth muscle. Science 180, 1332–1339 (1973).
Ross, R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 362, 801–809 (1993).
Ross, R. Atherosclerosis-An inflammatory disease. N. Engl. J. Med. 340, 115–126 (1999).
Kearney, M. et al. Histopathology of in-stent restenosis in patients with peripheral artery disease. Circulation 95, 1998–2002 (1997).
Billingham, M.E. Cardiac transplant atherosclerosis. Transplant. Proc. 19, 19–25 (1987).
Sata, M. et al. A mouse model of vascular injury that induces rapid onset of medial cell apoptosis followed by reproducible neointimal hyperplasia. J. Mol. Cell. Cardiol. 32, 2097–2104 (2000).
Feldman, L.J. et al. Differential expression of matrix metalloproteinases after stent implantation and balloon angioplasty in the hypercholesterolemic rabbit. Circulation 103, 3117–3122 (2001).
Roque, M. et al. Mouse model of femoral artery denudation injury associated with the rapid accumulation of adhesion molecules on the luminal surface and recruitment of neutrophils. Arterioscler. Thromb. Vasc. Biol. 20, 335–342 (2000).
Manka, D., Collins, R.G., Ley, K., Beaudet, A.L. & Sarembock, I.J. Absence of p-selectin, but not intercellular adhesion molecule-1, attenuates neointimal growth after arterial injury in apolipoprotein e-deficient mice. Circulation 103, 1000–1005 (2001).
Zohlnhofer, D. et al. Gene expression profiling of human stent-induced neointima by cDNA array analysis of microscopic specimens retrieved by helix cutter atherectomy: detection of FK506-binding protein 12 upregulation. Circulation 103, 1396–1402. (2001).
Zohlnhofer, D. et al. Transcriptome analysis reveals a role of interferon-gamma in human neointima formation. Mol Cell. 7, 1059–1069 (2001).
Miyamoto, T. et al. Expression of stem cell factor in human aortic endothelial and smooth muscle cells. Atherosclerosis. 129, 207–213 (1997).
Zambrowicz, B.P. et al. Disruption of overlapping transcripts in the ROSA βgeo 26 gene trap strain leads to widespread expression of β-galactosidase in mouse embryos and hematopoietic cells. Proc. Natl. Acad. Sci. USA 94, 3789–3794 (1997).
Saiura, A., Sata, M., Hirata, Y., Nagai, R. & Makuuchi, M. Circulating smooth muscle progenitor cells contribute to atherosclerosis. Nature Med. 7, 382–383 (2001).
Shimizu, K. et al. Host bone-marrow cells are a source of donor intimal smooth- muscle-like cells in murine aortic transplant arteriopathy. Nature Med. 7, 738–741 (2001).
Okabe, M., Ikawa, M., Kominami, K., Nakanishi, T. & Nishimune, Y. Green mice as a source of ubiquitous green cells. FEBS Lett. 407, 313–319 (1997).
Plump, A.S. et al. Sever hypercholesterolemia and atherosclerosis in Apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 71, 343–353 (1992).
Farb, A. et al. Pathology of acute and chronic coronary stenting in humans. Circulation 99, 44–52 (1999).
Schwartz, R.S. Pathophysiology of restenosis: interaction of thrombosis, hyperplasia, and/or remodeling. Am. J. Cardiol. 81, 14E–17E (1998).
Ross, R. Genetically modified mice as models of transplant atherosclerosis. Nature Med. 2, 527–528 (1996).
Sata, M. & Walsh, K. Endothelial cell apoptosis induced by oxidized LDL is associated with the downregulation of the cellular caspase inhibitor FLIP. J. Biol. Chem. 273, 33103–33106 (1998).
Tricot, O. et al. Relation between endothelial cell apoptosis and blood flow direction in human atherosclerotic plaques. Circulation 101, 2450–2453 (2000).
McKay, R. Stem cells-hype and hope. Nature 406, 361–364 (2000).
Orlic, D. et al. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc. Natl. Acad. Sci. USA 98, 10344–10349 (2001).
Asahara, T. et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 275, 964–967 (1997).
Walsh, K. & Perlman, H. Molecular strategies to inhibit restenosis: modulation of the vascular myocyte phenotype. Semin. Intervent. Cardiol. 1, 173–179 (1996).
Miller, A.M., McPhaden, A.R., Wadsworth, R.M. & Wainwright, C.L. Inhibition by leukocyte depletion of neointima formation after balloon angioplasty in a rabbit model of restenosis. Cardiovasc. Res. 49, 838–850 (2001).
Furukawa, Y. et al. Anti-monocyte chemoattractant protein-1/monocyte chemotactic and activating factor antibody inhibits neointimal hyperplasia in injured rat carotid arteries. Circ. Res. 84, 306–314 (1999).
Paris, F. et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science 293, 293–297 (2001).
Kaushal, S. et al. Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nature Med 7, 1035–1040 (2001).
Wright, D.E., Wagers, A.J., Gulati, A.P., Johnson, F.L. & Weissman, I.L. Physiological migration of hematopoietic stem and progenitor cells. Science 294, 1933–1936 (2001).
National Institutes of Health. Guide for the Care and Use of Laboratory Animals. NIH Publication No. 86-23, revised in 1985.
Okada, S. et al. Impairment of B lymphopoiesis in precocious aging (klotho) mice. Int. Immunol. 12, 861–871 (2000).
Corry, R.J., Winn, H.J. & Russell, P.S. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation 16, 343–350 (1973).
Perlman, H. et al. Bax-mediated cell death by the Gax homeoprotein requires mitogen-activation but is independent of cell cycle activity. EMBO J. 13, 3576–3586 (1998).
Tojo, A. et al. Immunohistochemical localization of multispecific renal organic anion transporter 1 in rat kidney. J. Am. Soc. Nephrol. 10, 464–471 (1999).
Acknowledgements
We thank H. Kato, T. Hasegawa, Y. Sugawara, M. Kinoshita and N. Nangi for technical assistance. This study was supported in part by grants from the Japan Heart Foundation, the Japan Foundation of Cardiovascular Research, the Naito Foundation, the Yamanouchi Foundation for Research on Metabolic Disorders, the Japan Research Foundation for Clinical Pharmacology, the NOVARTIS Foundation for the Promotion of Science, the Shionogi Foundation, the Asahi Glass Foundation, the Kanae foundation, the Takeda Medical Research Foundation, the Mitsukoshi health and welfare foundation and the Terumo Life Science Foundation (to S.M.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Sata, M., Saiura, A., Kunisato, A. et al. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat Med 8, 403–409 (2002). https://doi.org/10.1038/nm0402-403
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nm0402-403
This article is cited by
-
Mechanistic insights into the anti-restenotic effects of HSP27 and HO1 modulated by reconstituted HDL on neointimal hyperplasia
Scientific Reports (2023)
-
An update on the phenotypic switching of vascular smooth muscle cells in the pathogenesis of atherosclerosis
Cellular and Molecular Life Sciences (2022)
-
Bioinformatics Gene Analysis of Potential Biomarkers and Therapeutic Targets for Unstable Atherosclerotic Plaque-Related Stroke
Journal of Molecular Neuroscience (2021)
-
Silk fibroin vascular graft: a promising tissue-engineered scaffold material for abdominal venous system replacement
Scientific Reports (2020)
-
Modified method for effective primary vascular smooth muscle progenitor cell culture from peripheral blood
Cytotechnology (2020)