Abstract
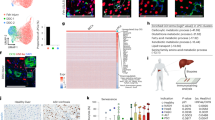
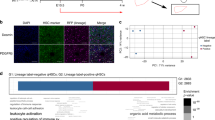
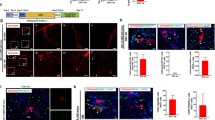
During chronic injury a population of bipotent hepatic progenitor cells (HPCs) become activated to regenerate both cholangiocytes and hepatocytes. Here we show in human diseased liver and mouse models of the ductular reaction that Notch and Wnt signaling direct specification of HPCs via their interactions with activated myofibroblasts or macrophages. In particular, we found that during biliary regeneration, expression of Jagged 1 (a Notch ligand) by myofibroblasts promoted Notch signaling in HPCs and thus their biliary specification to cholangiocytes. Alternatively, during hepatocyte regeneration, macrophage engulfment of hepatocyte debris induced Wnt3a expression. This resulted in canonical Wnt signaling in nearby HPCs, thus maintaining expression of Numb (a cell fate determinant) within these cells and the promotion of their specification to hepatocytes. By these two pathways adult parenchymal regeneration during chronic liver injury is promoted.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
15 March 2012
In the version of this article initially published online, the last sentence of the abstract incorrectly stated the study focused on a process involved in the recovery from acute liver injury when, in fact, chronic liver injury was meant. The error has been corrected for all versions of this article.
References
World Health Organization. Disease and injury country estimates, burden of disease http://www.who.int/healthinfo/global_burden_disease/estimates_country/en/index.html (2009).
Hay, D.C. Cadaveric hepatocytes repopulate diseased livers: life after death. Gastroenterology 139, 729–731 (2010).
Furuyama, K. et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat. Genet. 43, 34–41 (2010).
Fellous, T.G. et al. Locating the stem cell niche and tracing hepatocyte lineages in human liver. Hepatology 49, 1655–1663 (2009).
Gouw, A.S., Clouston, A.D. & Theise, N.D. Ductular reactions in human liver: diversity at the interface. Hepatology 54, 1853–1863 (2011).
Fallowfield, J.A. et al. Scar-associated macrophages are a major source of hepatic matrix metalloproteinase-13 and facilitate the resolution of murine hepatic fibrosis. J. Immunol. 178, 5288–5295 (2007).
Lin, S.L. et al. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc. Natl. Acad. Sci. USA 107, 4194–4199 (2010).
Duffield, J.S. et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J. Clin. Invest. 115, 56–65 (2005).
Tanimizu, N. & Miyajima, A. Notch signaling controls hepatoblast differentiation by altering the expression of liver-enriched transcription factors. J. Cell Sci. 117, 3165–3174 (2004).
Goessling, W. et al. APC mutant zebrafish uncover a changing temporal requirement for Wnt signaling in liver development. Dev. Biol. 320, 161–174 (2008).
Burke, Z.D. et al. Liver zonation occurs through a β-catenin-dependent, c-Myc-independent mechanism. Gastroenterology 136, 2316–2324 (2009).
Lozier, J., McCright, B. & Gridley, T. Notch signaling regulates bile duct morphogenesis in mice. PLoS ONE 3, e1851 (2008).
McCright, B., Lozier, J. & Gridley, T. A mouse model of Alagille syndrome: Notch2 as a genetic modifier of Jag1 haploinsufficiency. Development 129, 1075–1082 (2002).
Oda, T. et al. Mutations in the human Jagged1 gene are responsible for Alagille syndrome. Nat. Genet. 16, 235–242 (1997).
Li, L. et al. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat. Genet. 16, 243–251 (1997).
McDaniell, R. et al. NOTCH2 mutations cause Alagille syndrome, a heterogeneous disorder of the notch signaling pathway. Am. J. Hum. Genet. 79, 169–173 (2006).
Sparks, E.E., Huppert, K.A., Brown, M.A., Washington, M.K. & Huppert, S.S. Notch signaling regulates formation of the three-dimensional architecture of intrahepatic bile ducts in mice. Hepatology 51, 1391–1400 (2010).
Bray, S.J. Notch signalling: a simple pathway becomes complex. Nat. Rev. Mol. Cell Biol. 7, 678–689 (2006).
Iso, T., Kedes, L. & Hamamori, Y. HES and HERP families: multiple effectors of the Notch signaling pathway. J. Cell. Physiol. 194, 237–255 (2003).
Lee, Y.J., Swencki, B., Shoichet, S. & Shivdasani, R.A. A possible role for the high mobility group box transcription factor Tcf-4 in vertebrate gut epithelial cell differentiation. J. Biol. Chem. 274, 1566–1572 (1999).
Okamura, R.M. et al. Redundant regulation of T cell differentiation and TCRalpha gene expression by the transcription factors LEF-1 and TCF-1. Immunity 8, 11–20 (1998).
van Houte, L. et al. The sequence-specific high mobility group 1 box of TCF-1 adopts a predominantly α-helical conformation in solution. J. Biol. Chem. 268, 18083–18087 (1993).
McGill, M.A., Dho, S.E., Weinmaster, G. & McGlade, C.J. Numb regulates post-endocytic trafficking and degradation of Notch1. J. Biol. Chem. 284, 26427–26438 (2009).
McGill, M.A. & McGlade, C.J. Mammalian numb proteins promote Notch1 receptor ubiquitination and degradation of the Notch1 intracellular domain. J. Biol. Chem. 278, 23196–23203 (2003).
Spana, E.P. & Doe, C.Q. Numb antagonizes Notch signaling to specify sibling neuron cell fates. Neuron 17, 21–26 (1996).
Katoh, M. & Katoh, M. NUMB is a break of WNT-Notch signaling cycle. Int. J. Mol. Med. 18, 517–521 (2006).
Cheng, X., Huber, T.L., Chen, V.C., Gadue, P. & Keller, G.M. Numb mediates the interaction between Wnt and Notch to modulate primitive erythropoietic specification from the hemangioblast. Development 135, 3447–3458 (2008).
Cardinale, V. et al. Multipotent stem/progenitor cells in human biliary tree give rise to hepatocytes, cholangiocytes and pancreatic islets. Hepatology 54, 2159–2172 (2011).
Van Hul, N.K., Barca-Quinones, J., Sempoux, C., Horsmans, Y. & Leclercq, I.A. Relation between liver progenitor cell expansion and extracellular matrix deposition in a CDE-induced murine model of chronic liver injury. Hepatology 49, 1625–1635 (2009).
Akhurst, B. et al. A modified choline-deficient, ethionine-supplemented diet protocol effectively induces oval cells in mouse liver. Hepatology 34, 519–522 (2001).
Wang, X. et al. The origin and liver repopulating capacity of murine oval cells. Proc. Natl. Acad. Sci. USA 100 (suppl. 1), 11881–11888 (2003).
Fickert, P. et al. A new xenobiotic-induced mouse model of sclerosing cholangitis and biliary fibrosis. Am. J. Pathol. 171, 525–536 (2007).
Lorenzini, S. et al. Characterisation of a stereotypical cellular and extracellular adult liver progenitor cell niche in rodents and diseased human liver. Gut 59, 645–654 (2010).
van Es, J.H. et al. Notch/γ-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 435, 959–963 (2005).
Hellström, M. et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 445, 776–780 (2007).
Spee, B. et al. Characterisation of the liver progenitor cell niche in liver diseases: potential involvement of Wnt and Notch signalling. Gut 59, 247–257 (2010).
Tirnitz-Parker, J.E., Tonkin, J.N., Knight, B., Olynyk, J.K. & Yeoh, G.C. Isolation, culture and immortalisation of hepatic oval cells from adult mice fed a choline-deficient, ethionine-supplemented diet. Int. J. Biochem. Cell Biol. 39, 2226–2239 (2007).
Lustig, B. et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol. Cell. Biol. 22, 1184–1193 (2002).
Mori-Akiyama, Y. et al. SOX9 is required for the differentiation of paneth cells in the intestinal epithelium. Gastroenterology 133, 539–546 (2007).
Means, A.L., Xu, Y., Zhao, A., Ray, K.C. & Gu, G.A. CK19(CreERT) knockin mouse line allows for conditional DNA recombination in epithelial cells in multiple endodermal organs. Genesis 46, 318–323 (2008).
Chung, E.Y., Kim, S.J. & Ma, X.J. Regulation of cytokine production during phagocytosis of apoptotic cells. Cell Res. 16, 154–161 (2006).
Van Rooijen, N. & Sanders, A. Kupffer cell depletion by liposome-delivered drugs: comparative activity of intracellular clodronate, propamidine, and ethylenediaminetetraacetic acid. Hepatology 23, 1239–1243 (1996).
Lemaigre, F.P. Notch signaling in bile duct development: new insights raise new questions. Hepatology 48, 358–360 (2008).
Crosnier, C. et al. JAGGED1 gene expression during human embryogenesis elucidates the wide phenotypic spectrum of Alagille syndrome. Hepatology 32, 574–581 (2000).
Hofmann, J.J. et al. Jagged1 in the portal vein mesenchyme regulates intrahepatic bile duct development: insights into Alagille syndrome. Development 137, 4061–4072 (2010).
Yamasaki, H. et al. Suppression of C/EBPα expression in periportal hepatoblasts may stimulate biliary cell differentiation through increased Hnf6 and Hnf1b expression. Development 133, 4233–4243 (2006).
Clotman, F. et al. The onecut transcription factor HNF6 is required for normal development of the biliary tract. Development 129, 1819–1828 (2002).
Fletcher, J. et al. The inhibitory role of stromal cell mesenchyme on human embryonic stem cell hepatocyte differentiation is overcome by Wnt3a treatment. Cloning Stem Cells 10, 331–339 (2008).
Frise, E., Knoblich, J.A., Younger-Shepherd, S., Jan, L.Y. & Jan, Y.N. The Drosophila Numb protein inhibits signaling of the Notch receptor during cell-cell interaction in sensory organ lineage. Proc. Natl. Acad. Sci. USA 93, 11925–11932 (1996).
McGill, M.A. & McGlade, C.J. Mammalian numb proteins promote Notch1 receptor ubiquitination and degradation of the Notch1 intracellular domain. J. Biol. Chem. 278, 23196–23203 (2003).
Park, J. et al. Regulation of Sox9 by Sonic Hedgehog (Shh) is essential for patterning and formation of tracheal cartilage. Dev. Dyn. 239, 514–526 (2010).
Glise, B., Jones, D.L. & Ingham, P.W. Notch and Wingless modulate the response of cells to Hedgehog signalling in the Drosophila wing. Dev. Biol. 248, 93–106 (2002).
Omenetti, A. et al. The hedgehog pathway regulates remodelling responses to biliary obstruction in rats. Gut 57, 1275–1282 (2008).
Omenetti, A. & Diehl, A.M. Hedgehog signaling in cholangiocytes. Curr. Opin. Gastroenterol. 27, 268–275 (2011).
Choi, S.S., Omenetti, A., Syn, W.K. & Diehl, A.M. The role of Hedgehog signaling in fibrogenic liver repair. Int. J. Biochem. Cell Biol. 43, 238–244 (2011).
Karner, C.M. et al. Wnt9b signaling regulates planar cell polarity and kidney tubule morphogenesis. Nat. Genet. 41, 793–799 (2009).
Polakos, N.K. et al. Kupffer cell–dependent hepatitis occurs during influenza infection. Am. J. Pathol. 168, 1169–1178 (2006).
Klein, I. et al. Kupffer cell heterogeneity: functional properties of bone marrow derived and sessile hepatic macrophages. Blood 110, 4077–4085 (2007).
Reddy, S.M. et al. Phagocytosis of apoptotic cells by macrophages induces novel signaling events leading to cytokine-independent survival and inhibition of proliferation: activation of Akt and inhibition of extracellular signal-regulated kinases 1 and 2. J. Immunol. 169, 702–713 (2002).
Nijjar, S.S., Crosby, H.A., Wallace, L., Hubscher, S.G. & Strain, A.J. Notch receptor expression in adult human liver: a possible role in bile duct formation and hepatic neovascularization. Hepatology 34, 1184–1192 (2001).
Acknowledgements
Clodronate (Cl2MDP) was a gift of Roche Diagnostics (Mannheim, Germany). Thanks to R. Aucott (UK Medical Research Council (MRC)/University of Edinburgh Centre for Inflammation Research) for the donation of mouse hepatic fibroblasts. Krt19CreERT mice were a kind gift from G. Gu (Vanderbilt University), and the Ctnnb1ΔEx3 strain was provided by O. Sansom (The Beatson Institute for Cancer Research, Glasgow). S.J.F. is supported by the MRC, Sir Jules Thorn Trust and Wellcome Trust; L.G.B. is funded through an MRC project grant and was supported through an MRC studentship.
Author information
Authors and Affiliations
Contributions
L.B., O.G., T.G.B., P.R. and A.P. designed experiments and generated data, J.P.I., S.L., T.R. and S.J.F. designed experiments; S.S.S. generated the three-dimensional images; N.V.R. generated and supplied the clodronate liposomes; S.R., R.A.R. and B.S. generated data; L.B. and O.G. analyzed the data; L.B. prepared the manuscript; O.J.S., J.P.I., S.L., T.R. and S.J.F. edited the manuscript; J.P.I., T.R. and S.J.F. provided experimental funding.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Table 1 (PDF 1824 kb)
Rights and permissions
About this article
Cite this article
Boulter, L., Govaere, O., Bird, T. et al. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med 18, 572–579 (2012). https://doi.org/10.1038/nm.2667
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2667
This article is cited by
-
GITRL impairs hepatocyte repopulation by liver progenitor cells to aggravate inflammation and fibrosis by GITR+CD8+ T lymphocytes in CDE Mice
Cell Death & Disease (2024)
-
Current view of liver cancer cell-of-origin and proposed mechanisms precluding its proper determination
Cancer Cell International (2023)
-
β-adrenergic receptor agonist promotes ductular expansion during 3,5-diethoxycarbonyl-1,4-dihydrocollidine-induced chronic liver injury
Scientific Reports (2023)
-
Prednisolone and mesenchymal stem cell preloading protect liver cell migration and mitigate extracellular matrix modification in transplanted decellularized rat liver
Stem Cell Research & Therapy (2022)
-
Identification of a rare Gli1+ progenitor cell population contributing to liver regeneration during chronic injury
Cell Discovery (2022)