Abstract
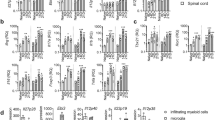
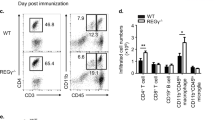
T helper cells that produce interleukin 17 (IL-17) are associated with inflammation and the control of certain bacteria. We report here the essential involvement of the adaptor protein Act1 in IL-17 receptor (IL-17R) signaling and IL-17-dependent immune responses. After stimulation with IL-17, recruitment of Act1 to IL-17R required the IL-17R conserved cytoplasmic 'SEFIR' domain, followed by recruitment of the kinase TAK1 and E3 ubiquitin ligase TRAF6, which mediate 'downstream' activation of transcription factor NF-κB. IL-17-induced expression of inflammation-related genes was abolished in Act1-deficient primary astroglial and gut epithelial cells. This reduction was associated with much less inflammatory disease in vivo in both autoimmune encephalomyelitis and dextran sodium sulfate–induced colitis. Our data show that Act1 is essential in IL-17-dependent signaling in autoimmune and inflammatory disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Kolls, J.K. & Linden, A. Interleukin-17 family members and inflammation. Immunity 21, 467–476 (2004).
Veldhoen, M., Hocking, R.J., Atkins, C.J., Locksley, R.M. & Stockinger, B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24, 179–189 (2006).
Mangan, P.R. et al. Transforming growth factor-β induces development of the TH17 lineage. Nature 441, 231–234 (2006).
Harrington, L.E. et al. Interleukin 17–producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6, 1123–1132 (2005).
Park, H. et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 6, 1133–1141 (2005).
Bettelli, E. et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 (2006).
Mangan, P.R. et al. Transforming growth factor-β induces development of the TH17 lineage. Nature 441, 231–234 (2006).
Nakae, S. et al. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity 17, 375–387 (2002).
Iwakura, Y. & Ishigame, H. The IL-23/IL-17 axis in inflammation. J. Clin. Invest. 116, 1218–1222 (2006).
Kolls, J.K. & Linden, A. Interleukin-17 family members and inflammation. Immunity 21, 467–476 (2004).
Toy, D. et al. Cutting Edge: Interleukin 17 signals through a heteromeric receptor complex. J. Immunol. 177, 36–39 (2006).
Trajkovic, V. et al. Interleukin-17 stimulates inducible nitric oxide synthase activation in rodent astrocytes. J. Neuroimmunol. 119, 183–191 (2001).
Inoue, D. et al. IL-17A promotes the growth of airway epithelial cells through ERK-dependent signaling pathway. Biochem. Biophys. Res. Commun. 347, 852–858 (2006).
Novatchkova, M., Leibbrandt, A., Werzowa, J., Neubuser, A. & Eisenhaber, F. The STIR-domain superfamily in signal transduction, development and immunity. Trends Biochem. Sci. 28, 226–229 (2003).
Yao, Z. et al. Molecular characterization of the human interleukin (IL)-17 receptor. Cytokine 9, 794–800 (1997).
Schwandner, R., Yamaguchi, K. & Cao, Z. Requirement of tumor necrosis factor receptor-associated factor (TRAF)6 in interleukin 17 signal transduction. J. Exp. Med. 191, 1233–1240 (2000).
Li, X. et al. Act1, an NF-κB-activating protein. Proc. Natl. Acad. Sci. USA 97, 10489–10493 (2000).
Leonardi, A., Chariot, A., Claudio, E., Cunningham, K. & Siebenlist, U. CIKS, a connection to IκB kinase and stress-activated protein kinase. Proc. Natl. Acad. Sci. USA 97, 10494–10499 (2000).
Qian, Y., Zhao, Z., Jiang, Z. & Li, X. Role of NFκB activator Act1 in CD40-mediated signaling in epithelial cells. Proc. Natl. Acad. Sci. USA 99, 9386–9391 (2002).
Qian, Y. et al. Act1, a negative regulator in CD40- and BAFF-mediated B cell survival. Immunity 21, 575–587 (2004).
Novatchkova, M., Leibbrandt, A., Werzowa, J., Neubuser, A. & Eisenhaber, F. The STIR-domain superfamily in signal transduction, development and immunity. Trends Biochem. Sci. 28, 226–229 (2003).
Cua, D.J. et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421, 744–748 (2003).
Chen, Y. et al. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J. Clin. Invest. 116, 1317–1326 (2006).
Park, H. et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 6, 1133–1141 (2005).
Chen, Y. et al. Stimulation of airway mucin gene expression by interleukin (IL)-17 through IL-6 paracrine/autocrine loop. J. Biol. Chem. 278, 17036–17043 (2003).
Awane, M., Andres, P.G., Li, D.J. & Reinecker, H.C. NF-κB-inducing kinase is a common mediator of IL-17-, TNF-α-, and IL-1β-induced chemokine promoter activation in intestinal epithelial cells. J. Immunol. 162, 5337–5344 (1999).
Ruddy, M.J. et al. Functional cooperation between interleukin-17 and tumor necrosis factor-α is mediated by CCAAT/enhancer-binding protein family members. J. Biol. Chem. 279, 2559–2567 (2004).
Weaver, A. et al. An elevated matrix metalloproteinase (MMP) in an animal model of multiple sclerosis is protective by affecting Th1/Th2 polarization. FASEB J. 19, 1668–1670 (2005).
Becher, B., Bechmann, I. & Greter, M. Antigen presentation in autoimmunity and CNS inflammation: how T lymphocytes recognize the brain. J. Mol. Med. 84, 532–543 (2006).
Gold, R., Linington, C. & Lassmann, H. Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain 129, 1953–1971 (2006).
Koenders, M.I. et al. Interleukin-17 receptor deficiency results in impaired synovial expression of interleukin-1 and matrix metalloproteinases 3, 9, and 13 and prevents cartilage destruction during chronic reactivated streptococcal cell wall-induced arthritis. Arthritis Rheum. 52, 3239–3247 (2005).
Dong, Y. & Benveniste, E.N. Immune function of astrocytes. Glia 36, 180–190 (2001).
Iwakura, Y. & Ishigame, H. The IL-23/IL-17 axis in inflammation. J. Clin. Invest. 116, 1218–1222 (2006).
Zhang, Z., Zheng, M., Bindas, J., Schwarzenberger, P. & Kolls, J.K. Critical role of IL-17 receptor signaling in acute TNBS-induced colitis. Inflamm. Bowel Dis. 12, 382–388 (2006).
Zhao, Z. et al. IFN regulatory factor-1 is required for the up-regulation of the CD40-NF-κB activator 1 axis during airway inflammation. J. Immunol. 170, 5674–5680 (2003).
Schwartz, S., Beaulieu, J.F. & Ruemmele, F.M. Interleukin-17 is a potent immuno-modulator and regulator of normal human intestinal epithelial cell growth. Biochem. Biophys. Res. Commun. 337, 505–509 (2005).
Wen, F. et al. Expression of conditional cre recombinase in epithelial tissues of transgenic mice. Genesis 35, 100–106 (2003).
Kolls, J.K. & Linden, A. Interleukin-17 family members and inflammation. Immunity 21, 467–476 (2004).
Ye, P. et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 194, 519–527 (2001).
Kullberg, M.C. et al. IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. J. Exp. Med. 203, 2485–2494 (2006).
Shen, F., Hu, Z., Goswami, J. & Gaffen, S.L. Identification of common transcriptional regulatory elements in interleukin-17 target genes. J. Biol. Chem. 281, 24138–24148 (2006).
Sato, S. et al. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat. Immunol. 6, 1087–1095 (2005).
Shim, J.H. et al. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 19, 2668–2681 (2005).
Park, H. et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 6, 1133–1141 (2005).
Weaver, A. et al. An elevated matrix metalloproteinase (MMP) in an animal model of multiple sclerosis is protective by affecting Th1/Th2 polarization. FASEB J. 19, 1668–1670 (2005).
Becher, B., Bechmann, I. & Greter, M. Antigen presentation in autoimmunity and CNS inflammation: how T lymphocytes recognize the brain. J. Mol. Med. 84, 532–543 (2006).
Gold, R., Linington, C. & Lassmann, H. Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain 129, 1953–1971 (2006).
Koenders, M.I. et al. Interleukin-17 receptor deficiency results in impaired synovial expression of interleukin-1 and matrix metalloproteinases 3, 9, and 13 and prevents cartilage destruction during chronic reactivated streptococcal cell wall-induced arthritis. Arthritis Rheum. 52, 3239–3247 (2005).
Liang, L., Porter, E.M. & Sha, W.C. Constitutive expression of the B7h ligand for inducible costimulator on naive B cells is extinguished after activation by distinct B cell receptor and interleukin 4 receptor-mediated pathways and can be rescued by CD40 signaling. J. Exp. Med. 196, 97–108 (2002).
Park, H. et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 6, 1133–1141 (2005).
Hjelmstrom, P., Juedes, A.E., Fjell, J. & Ruddle, N.H. B-cell-deficient mice develop experimental allergic encephalomyelitis with demyelination after myelin oligodendrocyte glycoprotein sensitization. J. Immunol. 161, 4480–4483 (1998).
Li, Q., Estepa, G., Memet, S., Israel, A. & Verma, I.M. Complete lack of NF-κB activity in IKK1 and IKK2 double-deficient mice: additional defect in neurulation. Genes Dev. 14, 1729–1733 (2000).
Lomaga, M.A. et al. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 13, 1015–1024 (1999).
Han, Y., Wang, J., Zhou, Z. & Ransohoff, R.M. TGFβ1 selectively up-regulates CCR1 expression in primary murine astrocytes. Glia 30, 1–10 (2000).
Kordula, T. et al. Oncostatin M and the interleukin-6 and soluble interleukin-6 receptor complex regulate α1-antichymotrypsin expression in human cortical astrocytes. J. Biol. Chem. 273, 4112–4118 (1998).
Acknowledgements
We thank R.G. Oshima (Burnham Institute for Medical Research) for K18-Cre mice; D. Cua (DNAX) for technical discussions of T cell transfer experiments; and C. Dong for discussions. Human cortical astrocyte cultures were provided by S. Wright (Elan Pharmaceuticals). Supported by the National Institutes of Health (AI 065470 to X.L.) and the Leukemia and Lymphoma Society (Y.Q.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Model of IL-17 mediated signaling. (PDF 73 kb)
Supplementary Table 1
Primers and oligonucleotides. (PDF 54 kb)
Rights and permissions
About this article
Cite this article
Qian, Y., Liu, C., Hartupee, J. et al. The adaptor Act1 is required for interleukin 17–dependent signaling associated with autoimmune and inflammatory disease. Nat Immunol 8, 247–256 (2007). https://doi.org/10.1038/ni1439
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1439
This article is cited by
-
Interleukins in Epilepsy: Friend or Foe
Neuroscience Bulletin (2024)
-
New insights into the function of Interleukin-25 in disease pathogenesis
Biomarker Research (2023)
-
NF-κB Activator 1 downregulation in macrophages activates STAT3 to promote adenoma-adenocarcinoma transition and immunosuppression in colorectal cancer
BMC Medicine (2023)
-
The IL-17 family in diseases: from bench to bedside
Signal Transduction and Targeted Therapy (2023)
-
IL-17 and IL-17-producing cells in protection versus pathology
Nature Reviews Immunology (2023)