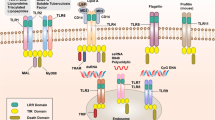
Abstract
C-type lectins expressed on myeloid cells comprise a family of proteins that share a common structural motif, and some act as receptors in pathogen recognition. But just as the presence of leucine-rich repeats alone is not sufficient to define a Toll-like receptor, the characterization of C-type lectin receptors in innate immunity requires the identification of accompanying signaling motifs. Here we focus on the known signaling pathways of myeloid C-type lectins and on their possible functions as autonomous activating or inhibitory receptors involved in innate responses to pathogens or self.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Janeway, C.A., Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 54, 1–13 (1989).
Kawai, T. & Akira, S. Innate immune recognition of viral infection. Nat. Immunol. 7, 131–137 (2006).
Medzhitov, R. & Janeway, C.A., Jr. Innate immunity: the virtues of a nonclonal system of recognition. Cell 91, 295–298 (1997).
Akira, S., Uematsu, S. & Takeuchi, O. Pathogen recognition and innate immunity. Cell 124, 783–801 (2006).
Crocker, P.R. Siglecs in innate immunity. Curr. Opin. Pharmacol. 5, 431–437 (2005).
Zelensky, A.N. & Gready, J.E. The C-type lectin-like domain superfamily. FEBS J 272, 6179–6217 (2005).
Geijtenbeek, T.B., van Vliet, S.J. & Engering, A., Hart, B.A. & van Kooyk, Y. Self- and nonself-recognition by C-type lectins on dendritic cells. Annu. Rev. Immunol. 22, 33–54 (2004).
Drickamer, K. C-type lectin-like domains. Curr. Opin. Struct. Biol. 9, 585–590 (1999).
O'Rourke, D., Baban, D., Demidova, M., Mott, R. & Hodgkin, J. Genomic clusters, putative pathogen recognition molecules, and antimicrobial genes are induced by infection of C. elegans with M. nematophilum. Genome Res. 16, 1005–1016 (2006).
Takahashi, K., Ip, W.E., Michelow, I.C. & Ezekowitz, R.A. The mannose-binding lectin: a prototypic pattern recognition molecule. Curr. Opin. Immunol. 18, 16–23 (2006).
Cash, H.L., Whitham, C.V., Behrendt, C.L. & Hooper, L.V. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 313, 1126–1130 (2006).
Lanier, L.L. NK cell recognition. Annu. Rev. Immunol. 23, 225–274 (2005).
Blander, J.M. & Medzhitov, R. Regulation of phagosome maturation by signals from Toll-like receptors. Science 304, 1014–1018 (2004).
Bonifacino, J.S. & Dell'Angelica, E.C. Molecular bases for the recognition of tyrosine-based sorting signals. J. Cell Biol. 145, 923–926 (1999).
Mahnke, K. et al. The dendritic cell receptor for endocytosis, DEC-205, can recycle and enhance antigen presentation via major histocompatibility complex class II-positive lysosomal compartments. J. Cell Biol. 151, 673–684 (2000).
Herre, J. et al. Dectin-1 uses novel mechanisms for yeast phagocytosis in macrophages. Blood 104, 4038–4045 (2004).
Rogers, N.C. et al. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity 22, 507–517 (2005).
Underhill, D.M., Rossnagle, E., Lowell, C.A. & Simmons, R.M. Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood 106, 2543–2550 (2005).
Gross, O. et al. Card9 controls a non-TLR signalling pathway for innate anti-fungal immunity. Nature 442, 651–656 (2006).
Engering, A. et al. The dendritic cell-specific adhesion receptor DC-SIGN internalizes antigen for presentation to T cells. J. Immunol. 168, 2118–2126 (2002).
Kwon, D.S., Gregorio, G., Bitton, N., Hendrickson, W.A. & Littman, D.R. DC-SIGN-mediated internalization of HIV is required for trans-enhancement of T cell infection. Immunity 16, 135–144 (2002).
Tacken, P.J., Torensma, R. & Figdor, C.G. Targeting antigens to dendritic cells in vivo. Immunobiology 211, 599–608 (2006).
Dzionek, A. et al. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon alpha/beta induction. J. Exp. Med. 194, 1823–1834 (2001).
Carrasco, Y.R. & Batista, F.D. B cell recognition of membrane-bound antigen: an exquisite way of sensing ligands. Curr. Opin. Immunol. 18, 286–291 (2006).
Brown, G.D. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat. Rev. Immunol. 6, 33–43 (2006).
Underhill, D.M. et al. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401, 811–815 (1999).
Edwards, A.D. et al. Microbial recognition via Toll-like receptor-dependent and -independent pathways determines the cytokine response of murine dendritic cell subsets to CD40 triggering. J. Immunol. 169, 3652–3660 (2002).
Steele, C. et al. The beta-glucan receptor dectin-1 recognizes specific morphologies of Aspergillus fumigatus. PLoS Pathog. 1, e42 (2005).
Hohl, T.M. et al. Aspergillus fumigatus triggers inflammatory responses by stage-specific β-glucan display. PLoS Pathog. 1, e30 (2005).
Dillon, S. et al. Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J. Clin. Invest. 116, 916–928 (2006).
Colonna, M., Samaridis, J. & Angman, L. Molecular characterization of two novel C-type lectin-like receptors, one of which is selectively expressed in human dendritic cells. Eur. J. Immunol. 30, 697–704 (2000).
Suzuki-Inoue, K. et al. A novel Syk-dependent mechanism of platelet activation by the C-type lectin receptor CLEC-2. Blood 107, 542–549 (2006).
Bakker, A.B., Baker, E., Sutherland, G.R., Phillips, J.H. & Lanier, L.L. Myeloid DAP12-associating lectin (MDL)-1 is a cell surface receptor involved in the activation of myeloid cells. Proc. Natl. Acad. Sci. USA 96, 9792–9796 (1999).
Kanazawa, N., Tashiro, K., Inaba, K. & Miyachi, Y. Dendritic cell immunoactivating receptor, a novel C-type lectin immunoreceptor, acts as an activating receptor through association with Fc receptor γ chain. J. Biol. Chem. 278, 32645–32652 (2003).
Ravetch, J.V. & Lanier, L.L. Immune inhibitory receptors. Science 290, 84–89 (2000).
Kanazawa, N. et al. DCIR acts as an inhibitory receptor depending on its immunoreceptor tyrosine-based inhibitory motif. J. Invest. Dermatol. 118, 261–266 (2002).
Richard, M., Thibault, N., Veilleux, P., Gareau-Page, G. & Beaulieu, A.D. Granulocyte macrophage-colony stimulating factor reduces the affinity of SHP-2 for the ITIM of CLECSF6 in neutrophils: a new mechanism of action for SHP-2. Mol. Immunol. 43, 1716–1721 (2006).
Marshall, A.S. et al. Identification and characterization of a novel human myeloid inhibitory C-type lectin-like receptor (MICL) that is predominantly expressed on granulocytes and monocytes. J. Biol. Chem. 279, 14792–14802 (2004).
Barrow, A.D. & Trowsdale, J. You say ITAM and I say ITIM, let's call the whole thing off: the ambiguity of immunoreceptor signalling. Eur. J. Immunol. 36, 1646–1653 (2006).
Hamerman, J.A. & Lanier, L.L. Inhibition of immune responses by ITAM-bearing receptors. Sci. STKE 2006, re1 (2006).
Chen, C.H. et al. Dendritic-cell-associated C-type lectin 2 (DCAL-2) alters dendritic-cell maturation and cytokine production. Blood 107, 1459–1467 (2006).
Geijtenbeek, T.B. et al. Mycobacteria target DC-SIGN to suppress dendritic cell function. J. Exp. Med. 197, 7–17 (2003).
Caparros, E. et al. DC-SIGN ligation on dendritic cells results in ERK and PI3K activation and modulates cytokine production. Blood 107, 3950–3958 (2006).
Gantner, B.N., Simmons, R.M., Canavera, S.J., Akira, S. & Underhill, D.M. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J. Exp. Med. 197, 1107–1117 (2003).
Brown, G.D. et al. Dectin-1 mediates the biological effects of β-glucans. J. Exp. Med. 197, 1119–1124 (2003).
Napolitani, G., Rinaldi, A., Bertoni, F., Sallusto, F. & Lanzavecchia, A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1–polarizing program in dendritic cells. Nat. Immunol. 6, 769–776 (2005).
Gautier, G. et al. A type I interferon autocrine-paracrine loop is involved in Toll-like receptor-induced interleukin-12p70 secretion by dendritic cells. J. Exp. Med. 201, 1435–1446 (2005).
Stahl, P., Schlesinger, P.H., Sigardson, E., Rodman, J.S. & Lee, Y.C. Receptor-mediated pinocytosis of mannose glycoconjugates by macrophages: characterization and evidence for receptor recycling. Cell 19, 207–215 (1980).
Ariizumi, K. et al. Identification of a novel, dendritic cell-associated molecule, dectin-1, by subtractive cDNA cloning. J. Biol. Chem. 275, 20157–20167 (2000).
Adachi, Y. et al. Characterization of β-glucan recognition site on C-type lectin, dectin 1. Infect. Immun. 72, 4159–4171 (2004).
Kim, Y.S. et al. Gram-negative bacteria-binding protein, a pattern recognition receptor for lipopolysaccharide and β-1,3-glucan that mediates the signaling for the induction of innate immune genes in Drosophila melanogaster cells. J. Biol. Chem. 275, 32721–32727 (2000).
Ochiai, M. & Ashida, M. A pattern-recognition protein for β-1,3-glucan. The binding domain and the cDNA cloning of β-1,3-glucan recognition protein from the silkworm, Bombyx mori. J. Biol. Chem. 275, 4995–5002 (2000).
Gantner, B.N., Simmons, R.M. & Underhill, D.M. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J. 24, 1277–1286 (2005).
Kang, P.B. et al. The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis. J. Exp. Med. 202, 987–999 (2005).
Bergman, M.P. et al. Helicobacter pylori modulates the T helper cell 1/T helper cell 2 balance through phase-variable interaction between lipopolysaccharide and DC-SIGN. J. Exp. Med. 200, 979–990 (2004).
Swain, S.D., Lee, S.J., Nussenzweig, M.C. & Harmsen, A.G. Absence of the macrophage mannose receptor in mice does not increase susceptibility to Pneumocystis carinii infection in vivo. Infect. Immun. 71, 6213–6221 (2003).
Lee, S.J., Zheng, N.Y., Clavijo, M. & Nussenzweig, M.C. Normal host defense during systemic candidiasis in mannose receptor-deficient mice. Infect. Immun. 71, 437–445 (2003).
Mi, Y., Shapiro, S.D. & Baenziger, J.U. Regulation of lutropin circulatory half-life by the mannose/N-acetylgalactosamine-4–SO4 receptor is critical for implantation in vivo. J. Clin. Invest. 109, 269–276 (2002).
van Gisbergen, K.P., Sanchez-Hernandez, M., Geijtenbeek, T.B. & van Kooyk, Y. Neutrophils mediate immune modulation of dendritic cells through glycosylation-dependent interactions between Mac-1 and DC-SIGN. J. Exp. Med. 201, 1281–1292 (2005).
van Gisbergen, K.P., Ludwig, I.S., Geijtenbeek, T.B. & van Kooyk, Y. Interactions of DC-SIGN with Mac-1 and CEACAM1 regulate contact between dendritic cells and neutrophils. FEBS Lett. 579, 6159–6168 (2005).
Lee, S.J. et al. Mannose receptor-mediated regulation of serum glycoprotein homeostasis. Science 295, 1898–1901 (2002).
Oka, K. et al. Lectin-like oxidized low-density lipoprotein receptor 1 mediates phagocytosis of aged/apoptotic cells in endothelial cells. Proc. Natl. Acad. Sci. USA 95, 9535–9540 (1998).
Yuita, H. et al. Retardation of removal of radiation-induced apoptotic cells in developing neural tubes in macrophage galactose-type C-type lectin-1-deficient mouse embryos. Glycobiology 15, 1368–1375 (2005).
Delneste, Y. et al. Involvement of LOX-1 in dendritic cell-mediated antigen cross-presentation. Immunity 17, 353–362 (2002).
van Gisbergen, K.P., Aarnoudse, C.A., Meijer, G.A., Geijtenbeek, T.B. & van Kooyk, Y. Dendritic cells recognize tumor-specific glycosylation of carcinoembryonic antigen on colorectal cancer cells through dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin. Cancer Res. 65, 5935–5944 (2005).
van Vliet, S.J. et al. Carbohydrate profiling reveals a distinctive role for the C-type lectin MGL in the recognition of helminth parasites and tumor antigens by dendritic cells. Int. Immunol. 17, 661–669 (2005).
Yoshida, T. et al. SRCL/CL-P1 recognizes GalNAc and a carcinoma-associated antigen, Tn antigen. J. Biochem. 133, 271–277 (2003).
Aarnoudse, C.A., Garcia Vallejo, J.J., Saeland, E. & van Kooyk, Y. Recognition of tumor glycans by antigen-presenting cells. Curr. Opin. Immunol. 18, 105–111 (2006).
van Vliet, S.J., Gringhuis, S.I., Geijtenbeek, T.B. & van Kooyk, Y. Regulation of effector T cells by antigen-presenting cells via interaction of the C-type lectin MGL with CD45. Nat. Immunol. 7, 1200–1208 (2006).
Ryan, E.J. et al. Dendritic cell-associated lectin-1: a novel dendritic cell-associated, C-type lectin-like molecule enhances T cell secretion of IL-4. J. Immunol. 169, 5638–5648 (2002).
Aragane, Y. et al. Involvement of dectin-2 in ultraviolet radiation-induced tolerance. J. Immunol. 171, 3801–3807 (2003).
Chieppa, M. et al. Cross-linking of the mannose receptor on monocyte-derived dendritic cells activates an anti-inflammatory immunosuppressive program. J. Immunol. 171, 4552–4560 (2003).
Howard, M.J. & Isacke, C.M. The C-type lectin receptor Endo180 displays internalization and recycling properties distinct from other members of the mannose receptor family. J. Biol. Chem. 277, 32320–32331 (2002).
Bonifaz, L. et al. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J. Exp. Med. 196, 1627–1638 (2002).
van Kooyk, Y. & Geijtenbeek, T.B. DC-SIGN: escape mechanism for pathogens. Nat. Rev. Immunol. 3, 697–709 (2003).
Kang, Y.S. et al. SIGN-R1, a novel C-type lectin expressed by marginal zone macrophages in spleen, mediates uptake of the polysaccharide dextran. Int. Immunol. 15, 177–186 (2003).
Arce, I., Martinez-Munoz, L., Roda-Navarro, P. & Fernandez-Ruiz, E. The human C-type lectin CLECSF8 is a novel monocyte/macrophage endocytic receptor. Eur. J. Immunol. 34, 210–220 (2004).
Mc Dermott, R. et al. Birbeck granules are subdomains of endosomal recycling compartment in human epidermal Langerhans cells, which form where Langerin accumulates. Mol. Biol. Cell 13, 317–335 (2002).
Sato, K. et al. Redistributions of macrophages expressing the macrophage galactose-type C-type lectin (MGL) during antigen-induced chronic granulation tissue formation. Int. Immunol. 17, 559–568 (2005).
Allavena, P., Chieppa, M., Monti, P. & Piemonti, L. From pattern recognition receptor to regulator of homeostasis: the double-faced macrophage mannose receptor. Crit. Rev. Immunol. 24, 179–192 (2004).
Koppel, E.A., van Gisbergen, K.P., Geijtenbeek, T.B. & van Kooyk, Y. Distinct functions of DC-SIGN and its homologues L-SIGN (DC-SIGNR) and mSIGNR1 in pathogen recognition and immune regulation. Cell. Microbiol. 7, 157–165 (2005).
Koppel, E.A. et al. Specific ICAM-3 grabbing nonintegrin-related 1 (SIGNR1) expressed by marginal zone macrophages is essential for defense against pulmonary Streptococcus pneumoniae infection. Eur. J. Immunol. 35, 2962–2969 (2005).
Nagaoka, K. et al. Association of SIGNR1 with TLR4-MD-2 enhances signal transduction by recognition of LPS in gram-negative bacteria. Int. Immunol. 17, 827–836 (2005).
Kang, Y.S. et al. The C-type lectin SIGN-R1 mediates uptake of the capsular polysaccharide of Streptococcus pneumoniae in the marginal zone of mouse spleen. Proc. Natl. Acad. Sci. USA 101, 215–220 (2004).
McGreal, E.P. et al. The carbohydrate-recognition domain of Dectin-2 is a C-type lectin with specificity for high mannose. Glycobiology 16, 422–430 (2006).
Stambach, N.S. & Taylor, M.E. Characterization of carbohydrate recognition by langerin, a C-type lectin of Langerhans cells. Glycobiology 13, 401–410 (2003).
Tada, Y. et al. Identification and characterization of endogenous Langerin ligands in murine extracellular matrix. J. Invest. Dermatol. 126, 1549–1558 (2006).
Turville, S., Wilkinson, J., Cameron, P., Dable, J. & Cunningham, A.L. The role of dendritic cell C-type lectin receptors in HIV pathogenesis. J. Leukoc. Biol. 74, 710–718 (2003).
Kumamoto, Y. et al. Identification of sialoadhesin as a dominant lymph node counter-receptor for mouse macrophage galactose-type C-type lectin 1. J. Biol. Chem. 279, 49274–49280 (2004).
Takada, A. et al. Human macrophage C-type lectin specific for galactose and N-acetylgalactosamine promotes filovirus entry. J. Virol. 78, 2943–2947 (2004).
Tsuiji, M. et al. Molecular cloning and characterization of a novel mouse macrophage C-type lectin, mMGL2, which has a distinct carbohydrate specificity from mMGL1. J. Biol. Chem. 277, 28892–28901 (2002).
Palma, A.S. et al. Ligands for the β-glucan receptor, Dectin-1, assigned using “designer” microarrays of oligosaccharide probes (neoglycolipids) generated from glucan polysaccharides. J. Biol. Chem. 281, 5771–5779 (2006)
Acknowledgements
We thank members of the Immunobiology Laboratory (Cancer Research UK, London Research Institute) for discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Robinson, M., Sancho, D., Slack, E. et al. Myeloid C-type lectins in innate immunity. Nat Immunol 7, 1258–1265 (2006). https://doi.org/10.1038/ni1417
Issue Date:
DOI: https://doi.org/10.1038/ni1417
This article is cited by
-
Binding of Trichinella spiralis C-type lectin with syndecan-1 on intestinal epithelial cells mediates larval invasion of intestinal epithelium
Veterinary Research (2023)
-
Pattern recognition receptors in health and diseases
Signal Transduction and Targeted Therapy (2021)
-
Tri-mannose grafting of chitosan nanocarriers remodels the macrophage response to bacterial infection
Journal of Nanobiotechnology (2019)
-
Targeting CLL-1 for acute myeloid leukemia therapy
Journal of Hematology & Oncology (2019)
-
Phospholipase Cγ2 is critical for Ca2+ flux and cytokine production in anti-fungal innate immunity of human corneal epithelial cells
BMC Ophthalmology (2018)