Abstract
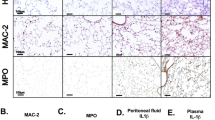
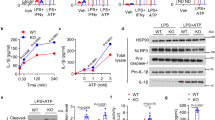
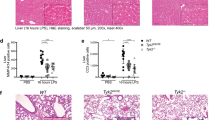
Autophagy, a cellular process for organelle and protein turnover, regulates innate immune responses. Here we demonstrate that depletion of the autophagic proteins LC3B and beclin 1 enhanced the activation of caspase-1 and secretion of interleukin 1β (IL-1β) and IL-18. Depletion of autophagic proteins promoted the accumulation of dysfunctional mitochondria and cytosolic translocation of mitochondrial DNA (mtDNA) in response to lipopolysaccharide (LPS) and ATP in macrophages. Release of mtDNA into the cytosol depended on the NALP3 inflammasome and mitochondrial reactive oxygen species (ROS). Cytosolic mtDNA contributed to the secretion of IL-1β and IL-18 in response to LPS and ATP. LC3B-deficient mice produced more caspase-1-dependent cytokines in two sepsis models and were susceptible to LPS-induced mortality. Our study suggests that autophagic proteins regulate NALP3-dependent inflammation by preserving mitochondrial integrity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
He, C. & Klionsky, D.J. Regulation mechanisms and signaling pathways of autophagy. Annu. Rev. Genet. 43, 67–93 (2009).
Levine, B. & Kroemer, G. Autophagy in the pathogenesis of disease. Cell 132, 27–42 (2008).
Virgin, H.W. & Levine, B. Autophagy genes in immunity. Nat. Immunol. 10, 461–470 (2009).
Saitoh, T. et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1β production. Nature 456, 264–268 (2008).
Stutz, A., Golenbock, D.T. & Latz, E. Inflammasomes: too big to miss. J. Clin. Invest. 119, 3502–3511 (2009).
Schroder, K. & Tschopp, J. The inflammasomes. Cell 140, 821–832 (2010).
Franchi, L., Eigenbrod, T., Munoz-Planillo, R. & Nunez, G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat. Immunol. 10, 241–247 (2009).
Li, P. et al. Mice deficient in IL-1β-converting enzyme are defective in production of mature IL-1β and resistant to endotoxic shock. Cell 80, 401–411 (1995).
Sutterwala, F.S. et al. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity 24, 317–327 (2006).
Aoki, H. et al. Monitoring autophagy in glioblastoma with antibody against isoform B of human microtubule-associated protein 1 light chain 3. Autophagy 4, 467–475 (2008).
Qu, X. et al. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J. Clin. Invest. 112, 1809–1820 (2003).
Qu, Y., Franchi, L., Nunez, G. & Dubyak, G.R. Nonclassical IL-1β secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J. Immunol. 179, 1913–1925 (2007).
Tal, M.C. et al. Absence of autophagy results in reactive oxygen species-dependent amplification of RLR signaling. Proc. Natl. Acad. Sci. USA 106, 2770–2775 (2009).
Li, N. et al. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J. Biol. Chem. 278, 8516–8525 (2003).
DiMauro, S. & Schon, E.A. Mitochondrial respiratory-chain diseases. N. Engl. J. Med. 348, 2656–2668 (2003).
Liesa, M., Palacin, M. & Zorzano, A. Mitochondrial dynamics in mammalian health and disease. Physiol. Rev. 89, 799–845 (2009).
Kroemer, G., Galluzzi, L. & Brenner, C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 87, 99–163 (2007).
Hashiguchi, K. & Zhang-Akiyama, Q.M. Establishment of human cell lines lacking mitochondrial DNA. Methods Mol. Biol. 554, 383–391 (2009).
King, M.P. & Attardi, G. Human cells lacking mtDNA: repopulation with exogenous mitochondria by complementation. Science 246, 500–503 (1989).
Chandel, N.S. & Schumacker, P.T. Cells depleted of mitochondrial DNA (rho0) yield insight into physiological mechanisms. FEBS Lett. 454, 173–176 (1999).
Trnka, J., Blaikie, F.H., Logan, A., Smith, R.A. & Murphy, M.P. Antioxidant properties of MitoTEMPOL and its hydroxylamine. Free Radic. Res. 43, 4–12 (2009).
Jiang, J. et al. A mitochondria-targeted triphenylphosphonium-conjugated nitroxide functions as a radioprotector/mitigator. Radiat. Res. 172, 706–717 (2009).
Rathinam, V.A. et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat. Immunol. 11, 395–402 (2010).
Fernandes-Alnemri, T. et al. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat. Immunol. 11, 385–393 (2010).
Jones, J.W. et al. Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proc. Natl. Acad. Sci. USA 107, 9771–9776 (2010).
Lemasters, J.J., Theruvath, T.P., Zhong, Z. & Nieminen, A.L. Mitochondrial calcium and the permeability transition in cell death. Biochim. Biophys. Acta 1787, 1395–1401 (2009).
Zhang, Q. et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464, 104–107 (2010).
Zhou, R., Tardivel, A., Thorens, B., Choi, I. & Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 11, 136–140 (2010).
Lamkanfi, M. et al. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J. Cell Biol. 187, 61–70 (2009).
Juliana, C. et al. Anti-inflammatory compounds parthenolide and Bay 11–7082 are direct inhibitors of the inflammasome. J. Biol. Chem. 285, 9792–9802 (2010).
Fahy, R.J. et al. Inflammasome mRNA expression in human monocytes during early septic shock. Am. J. Respir. Crit. Care Med. 177, 983–988 (2008).
Mortensen, M. et al. Loss of autophagy in erythroid cells leads to defective removal of mitochondria and severe anemia in vivo. Proc. Natl. Acad. Sci. USA 107, 832–837 (2010).
Cruz, C.M. et al. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J. Biol. Chem. 282, 2871–2879 (2007).
Adinolfi, E. et al. Basal activation of the P2X7 ATP receptor elevates mitochondrial calcium and potential, increases cellular ATP levels, and promotes serum-independent growth. Mol. Biol. Cell 16, 3260–3272 (2005).
Garcia-Marcos, M. et al. Role of sodium in mitochondrial membrane depolarization induced by P2X7 receptor activation in submandibular glands. FEBS Lett. 579, 5407–5413 (2005).
Dostert, C. et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320, 674–677 (2008).
Unterholzner, L. et al. IFI16 is an innate immune sensor for intracellular DNA. Nat. Immunol. 11, 997–1004 (2010).
Hornung, V. & Latz, E. Intracellular DNA recognition. Nat. Rev. Immunol. 10, 123–130 (2010).
Watanabe, E. et al. Sepsis induces extensive autophagic vacuolization in hepatocytes: a clinical and laboratory-based study. Lab. Invest. 89, 549–561 (2009).
Fredriksson, K. et al. Dysregulation of mitochondrial dynamics and the muscle transcriptome in ICU patients suffering from sepsis induced multiple organ failure. PLoS ONE 3, e3686 (2008).
d'Avila, J.C. et al. Sepsis induces brain mitochondrial dysfunction. Crit. Care Med. 36, 1925–1932 (2008).
Cann, G.M. et al. Developmental expression of LC3α and β: absence of fibronectin or autophagy phenotype in LC3β knockout mice. Dev. Dyn. 237, 187–195 (2008).
Wang, X.M., Kim, H.P., Nakahira, K., Ryter, S.W. & Choi, A.M. The heme oxygenase-1/carbon monoxide pathway suppresses TLR4 signaling by regulating the interaction of TLR4 with caveolin-1. J. Immunol. 182, 3809–3818 (2009).
Meissner, F., Molawi, K. & Zychlinsky, A. Superoxide dismutase 1 regulates caspase-1 and endotoxic shock. Nat. Immunol. 9, 866–872 (2008).
Chen, Z.H. et al. Egr-1 regulates autophagy in cigarette smoke-induced chronic obstructive pulmonary disease. PLoS ONE 3, e3316 (2008).
Eaton, J.S., Lin, Z.P., Sartorelli, A.C., Bonawitz, N.D. & Shadel, G.S. Ataxia-telangiectasia mutated kinase regulates ribonucleotide reductase and mitochondrial homeostasis. J. Clin. Invest. 117, 2723–2734 (2007).
Pelegrin, P., Barroso-Gutierrez, C. & Surprenant, A. P2X7 receptor differentially couples to distinct release pathways for IL-1β in mouse macrophage. J. Immunol. 180, 7147–7157 (2008).
Chung, S.W., Liu, X., Macias, A.A., Baron, R.M. & Perrella, M.A. Heme oxygenase-1-derived carbon monoxide enhances the host defense response to microbial sepsis in mice. J. Clin. Invest. 118, 239–247 (2008).
Acknowledgements
We thank B. Levine (University of Texas Southwestern Medical Center) for Becn1+/− mice; and E. Ifedigbo for technical assistance. Supported by National Institutes of Health (HL079904-12, HL08554, HL060234-10 and HL097005 to A.M.K.C., and AI083713 and AI067497 to K.A.F.) and the New England Regional Center of Excellence for Biodefense and Emerging Infectious Diseases (National Institute of Allergy and Infectious Diseases of the National Institutes of Health; AI057159 to V.A.K.R).
Author information
Authors and Affiliations
Contributions
K.N., H.P.K., J.A.H and A.M.K.C conceived of the study with assistance from S.W.R. and K.A.F.; M.R. supervised the generation of LC3B-deficent mice; K.A.F. supervised the generation of AIM-2 deficient mice; K.N., S.J.L. and V.A.K.R did the in vitro experiments; J.A.H., S.J.L. and J.A.E. did the in vivo experiments; H.C.L. did the transmission electron microscopy; M.C. and K.N. did flow cytometry; T.D. analyzed human samples; K.N., J.A.H., A.M.K.C. and S.W.R. wrote the paper; and A.M.K.C supervised the entire project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Table 1 (PDF 5328 kb)
Rights and permissions
About this article
Cite this article
Nakahira, K., Haspel, J., Rathinam, V. et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol 12, 222–230 (2011). https://doi.org/10.1038/ni.1980
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1980
This article is cited by
-
The role of the NLRP3 inflammasome and pyroptosis in cardiovascular diseases
Nature Reviews Cardiology (2024)
-
The NLRP3 inflammasome: a vital player in inflammation and mediating the anti-inflammatory effect of CBD
Inflammation Research (2024)
-
Resveratrol and quercetin protect from Benzo(a)pyrene-induced autophagy in retinal pigment epithelial cells
International Ophthalmology (2024)
-
Circulating cell-free mitochondrial DNA levels and glucocorticoid sensitivity in a cohort of male veterans with and without combat-related PTSD
Translational Psychiatry (2024)
-
Crosstalk between autophagy and insulin resistance: evidence from different tissues
European Journal of Medical Research (2023)