Abstract
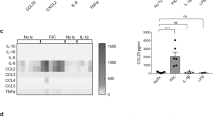
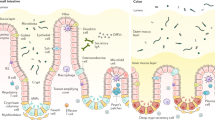
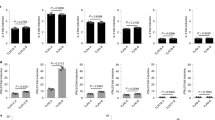
The mechanisms by which commensal bacteria suppress inflammatory signalling in the gut are still unclear. Here, we present a cellular mechanism whereby the polarity of intestinal epithelial cells (IECs) has a major role in colonic homeostasis. TLR9 activation through apical and basolateral surface domains have distinct transcriptional responses, evident by NF-κB activation and cDNA microarray analysis. Whereas basolateral TLR9 signals IκBα degradation and activation of the NF-κB pathway, apical TLR9 stimulation invokes a unique response in which ubiquitinated IκB accumulates in the cytoplasm preventing NF-κB activation. Furthermore, apical TLR9 stimulation confers intracellular tolerance to subsequent TLR challenges. IECs in TLR9-deficient mice, when compared with wild-type and TLR2-deficient mice, display a lower NF-κB activation threshold and these mice are highly susceptible to experimental colitis. Our data provide a case for organ-specific innate immunity in which TLR expression in polarized IECs has uniquely evolved to maintain colonic homeostasis and regulate tolerance and inflammation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Kazmierczak, B.I., Mostov, K. & Engel, J.N. Interaction of bacterial pathogens with polarized epithelium. Annu. Rev. Microbiol. 55, 407–435 (2001).
Naidu, A.S., Bidlack, W.R. & Clemens, R.A. Probiotic spectra of lactic acid bacteria (LAB). Crit. Rev. Food Sci. Nutr. 39, 13–126 (1999).
Bantel, H., Schmitz, M.L., Raible, A., Gregor, M. & Schulze-Osthoff, K. Critical role of NF-κB and stress-activated protein kinases in steroid unresponsiveness. FASEB. J. 16, 1832–1834 (2002).
Rachmilewitz, D. et al. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology 126, 520–528 (2004).
Rakoff-Nahoum, S., Paglino, J., Eslami-Varzaneh, F., Edberg, S. & Medzhitov, R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118, 229–241 (2004).
Rachmilewitz, D. et al. Immunostimulatory DNA ameliorates experimental and spontaneous murine colitis. Gastroenterology 122, 1428–1441 (2002).
Fukata, M. et al. Toll-like receptor-4 is required for intestinal response to epithelial injury and limiting bacterial translocation in a murine model of acute colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 288, G1055–G1065 (2005).
Katakura, K. et al. Toll-like receptor 9-induced type I IFN protects mice from experimental colitis. J. Clin. Invest. 115, 695–702 (2005).
Akira, S. & Hemmi, H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol. Lett. 85, 85–95 (2003).
Akira, S. & Takeda, K. Toll-like receptor signalling. Nature Rev. Immunol. 4, 499–511 (2004).
Cario, E. et al. Commensal-associated molecular patterns induce selective toll-like receptor-trafficking from apical membrane to cytoplasmic compartments in polarized intestinal epithelium. Am. J. Pathol. 160, 165–173 (2002).
Ortega-Cava, C.F. et al. Strategic compartmentalization of Toll-like receptor 4 in the mouse gut. J. Immunol. 170, 3977–3985 (2003).
Gewirtz, A.T. Navas,T.A., Lyons, S., Godowski,P.J. & Madara, J.L. Cutting edge: bacterial flagellin activates basolaterally expressed tlr5 to induce epithelial proinflammatory gene expression. J. Immunol. 167, 1882–1885 (2001).
Akhtar, M., Watson, J.L., Nazli, A. & McKay, D.M. Bacterial DNA evokes epithelial IL-8 production by a MAPK-dependent, NF-κB-independent pathway. FASEB J. 17, 1319–1321 (2003).
Otte, J.M., Cario, E. & Podolsky, D.K. Mechanisms of cross hyporesponsiveness to Toll-like receptor bacterial ligands in intestinal epithelial cells. Gastroenterology 126, 1054–1070 (2004).
Pedersen, G., Andresen, L., Matthiessen, M.W., Rask-Madsen, J. & Brynskov, J. Expression of Toll-like receptor 9 and response to bacterial CpG oligodeoxynucleotides in human intestinal epithelium. Clin. Exp. Immunol. 141, 298–306 (2005).
Katz, K.D. et al. Intestinal permeability in patients with Crohn's disease and their healthy relatives. Gastroenterology 97, 927–931 (1989).
Sandle, G.I. et al. Cellular basis for defective electrolyte transport in inflamed human colon. Gastroenterology 99, 97–105 (1990).
Latz, E. et al. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nature Immunol. 5, 190–198 (2004).
Leifer, C.A. et al. TLR9 is localized in the endoplasmic reticulum prior to stimulation. J. Immunol. 173, 1179–1183 (2004).
Okabe, Y., Kawane, K., Akira, S., Taniguchi, T. & Nagata, S. Toll-like receptor-independent gene induction program activated by mammalian DNA escaped from apoptotic DNA degradation. J. Exp. Med. 202, 1333–1339 (2005).
Cohen, S., Achbert-Weiner, H. & Ciechanover, A. Dual effects of IκB kinase β-mediated phosphorylation on p105 Fate: SCF(β-TrCP)-dependent degradation and SCF(β-TrCP)-independent processing. Mol. Cell. Biol. 24, 475–486 (2004).
Beinke, S., Robinson, M.J., Hugunin, M. & Ley, S.C. Lipopolysaccharide activation of the TPL-2/MEK/extracellular signal-regulated kinase mitogen-activated protein kinase cascade is regulated by IκB kinase-induced proteolysis of NF-κB1 p105. Mol. Cell. Biol. 24, 9658–9667 (2004).
Waterfield, M.R., Zhang, M., Norman, L.P. & Sun, S.C. NF-κB1/p105 regulates lipopolysaccharide-stimulated MAP kinase signaling by governing the stability and function of the Tpl2 kinase. Mol. Cell 11, 685–694 (2003).
van Es, J.H. et al. Wnt signalling induces maturation of Paneth cells in intestinal crypts. Nature Cell Biol. 7, 381–386 (2005).
Cooper, H.S., Murthy S.N., Shah, R.S. & Sedergran, D.J. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab. Invest. 69, 238–249 (1993).
Marrero, J.A., Matkowskyj, K.A., Yung, K., Hecht, G. & Benya, R.V. Dextran sulfate sodium-induced murine colitis activates NF-κB and increases galanin-1 receptor expression. Am. J. Physiol. Gastrointest. Liver Physiol. 278, G797–G804 (2000).
Rhee, S.H. et al. Pathophysiological role of Toll-like receptor 5 engagement by bacterial flagellin in colonic inflammation. Proc. Nat Acad. Sci. USA 102, 13610–13615 (2005).
Schmausser, B. et al. Expression and subcellular distribution of toll-like receptors TLR4, TLR5 and TLR9 on the gastric epithelium in Helicobacter pylori infection. Clin. Exp. Immunol. 136, 521–526 (2004).
Blitzer, J.T. & Nusse, R. A critical role for endocytosis in Wnt signaling. BMC. Cell Biol. 7, 28 (2006).
Van, I.S.C., Maier, O., Van Der Wouden, J.M. & Hoekstra, D. The subapical compartment and its role in intracellular trafficking and cell polarity. J. Cell Physiol. 184, 151–160 (2000).
Farquhar, M.G. & Palade, G.E. Junctional complexes in various epithelia. J. Cell Biol. 17, 375–412 (1963).
Beinke, S. et al. NF-κB1 p105 negatively regulates TPL-2 MEK kinase activity. Mol. Cell Biol. 23, 4739–4752 (2003).
Shang, F. et al. Lys6-modified ubiquitin inhibits ubiquitin-dependent protein degradation. J. Biol. Chem. 280, 20365–20374 (2005).
Neish, A.S. et al. Prokaryotic regulation of epithelial responses by inhibition of IκBα ubiquitination. Science 289, 1560–1563 (2000).
Kelly, D. et al. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-γ and RelA. Nature Immunol. 5, 104–112 (2004).
Campbell, A. Comparative molecular biology of lambdoid phages. Annu. Rev. Microbiol. 48, 193–222 (1994).
Parma, D.H. et al. The Rex system of bacteriophage lambda: tolerance and altruistic cell death. Genes Dev. 6, 497–510 (1992).
Bonev, M.N., Kozubek, S., Krasavin, E.A. & Amirtajev, K.G. λ-prophage induction in repair-deficient and wild type E. coli strains by γ-rays and heavy ions. Int. J. Radiat. Biol. 57, 993–1005 (1990).
Lee, J. et al. Molecular basis for the immunostimulatory activity of guanine nucleoside analogs: activation of Toll-like receptor 7. Proc. Natl Acad. Sci. USA 100, 6646–6651 (2003).
Greten, F.R. et al. IKKβ links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 118, 285–296 (2004).
Dwinell, M.B., Eckmann, L., Leopard, J.D., Varki, N.M. & Kagnoff, M.F. Chemokine receptor expression by human intestinal epithelial cells. Gastroenterology 117, 359–367 (1999).
Lee, J., Mira-Arbibe, L. & Ulevitch, R.J. TAK1 regulates multiple protein kinase cascades activated by bacterial lipopolysaccharide. J. Leukoc. Biol. 68, 909–915 (2000).
O'Neil, D.A. et al. Expression and regulation of the human β-defensins hBD-1 and hBD-2 in intestinal epithelium. J. Immunol. 163, 6718–6724 (1999).
Bozdech, Z. et al. Expression profiling of the schizont and trophozoite stages of Plasmodium falciparum with a long-oligonucleotide microarray. Genome Biol 4, R9 (2003).
Barczak, A. et al. Spotted long oligonucleotide arrays for human gene expression anasis. Genome Res 13, 1775–1785 (2003).
Acknowledgements
We thank P. Lee, J. Carmen, N. Varki, L. She, C. Yoon and M. Chung for technical assistance, S. Akira for TLR-null mice, and L. Beck and H. Cottam for critical reading of the manuscript. We also would like to thank B. Brinkman for his assistance in confocal imaging (UCSD Neuroscience Microscopy Shared Facility, NINDS grant P30 NS047101). This work is supported by National Institutes of Health (NIH) grants AI57709, DK35108 and AI40682 (E.R.), AI56075 and RR17030 (L.E.) European Commission (Program 6) Network of Excellence RUBICON and Israel Science Foundation-Centers of Excellence Program (Y.B.-N.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary figures S1, S2, S3, Supplementary tables S1 and S2 (PDF 709 kb)
Rights and permissions
About this article
Cite this article
Lee, J., Mo, JH., Katakura, K. et al. Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nat Cell Biol 8, 1327–1336 (2006). https://doi.org/10.1038/ncb1500
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1500
This article is cited by
-
Increased Intestinal Permeability: An Avenue for the Development of Autoimmune Disease?
Exposure and Health (2024)
-
Phenotypic heterogeneity in psoriatic arthritis: towards tissue pathology-based therapy
Nature Reviews Rheumatology (2023)
-
Role of mucosal immunity and epithelial–vascular barrier in modulating gut homeostasis
Internal and Emergency Medicine (2023)
-
Intestinal serotonergic system is modulated by Toll-like receptor 9
Journal of Physiology and Biochemistry (2022)
-
Gastrointestinal epithelial innate immunity—regionalization and organoids as new model
Journal of Molecular Medicine (2021)