Abstract
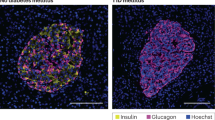
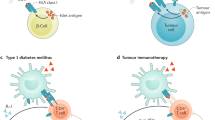
The hallmark of type 1 diabetes is specific destruction of pancreatic islet β-cells. Apoptosis of β-cells may be crucial at several points during disease progression, initiating leukocyte invasion of the islets and terminating the production of insulin in islet cells. β-Cell apoptosis may also be involved in the occasional evolution of type 2 into type 1 diabetes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Tisch, R. & McDevitt, H. Insulin-dependent diabetes mellitus. Cell 85, 291–297 (1996).
Bach, J. F. & Mathis, D. 70th forum in immunology: the NOD mouse. Res. Immunol. 148, 281–370 (1997).
Hoglund, P. et al. Initiation of autoimmune diabetes by developmentally regulated presentation of islet cell antigens in the pancreatic lymph nodes. J. Exp. Med. 189, 331–339 (1999).
Green, E. A., Eynon, E. E. & Flavell, R. A. Local expression of TNFα in neonatal NOD mice promotes diabetes by enhancing presentation of islet antigens. Immunity 9, 733–743 (1998).
Katz, J. D., Benoist, C. & Mathis, D. T helper cell subsets in insulin-dependent diabetes. Science 268, 1185–1188 (1995).
Gonzalez, A. et al. Genetic control of diabetes progression. Immunity 7, 873–883 (1997).
Finegood, D. T., Scaglia, L. & Bonner-Weir, S. Dynamics of β-cell mass in the growing rat pancreas. Diabetes 44, 249–256 (1995).
Scaglia, L., Cahill, C. J., Finegood, D. T. & Bonner-Weir, S. Apoptosis participates in the remodeling of the endocrine pancreas in the neonatal rat. Endocrinology 138, 1736–1741 (1997).
Petrik, J., Arany, E., McDonald, T. J. & Hill, D. J. Apoptosis in the pancreatic islet cells of the neonatal rat is associated with a reduced expression of insulin-like growth factor II that may act as a survival factor. Endocrinology 139, 2994–3004 (1998).
Trudeau, J. D. et al. Neonatal β-cell apoptosis: a trigger for autoimmune diabetes? Diabetes 49, 1–7 (2000).
Kassem, S. A., Ariel, I., Thornton, P. S., Scheimberg, I. & Glaser, B. β-cell proliferation and apoptosis in the developing normal human pancreas and in hyperinsulinism of infancy. Diabetes 49, 1325–1333 (2000).
Steinman, R. M., Turley, S., Mellman, I. & Inaba, K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J. Exp. Med. 191, 411–416 (2000).
Bellone, M. et al. Processing of engulfed apoptotic bodies yields T cell epitopes. J. Immunol. 159, 5391–5399 (1997).
Pittoni, V. & Isenberg, D. Apoptosis and antiphospholipid antibodies. Semin. Arthritis Rheum. 28, 163–178 (1998).
Coles, H. S. R., Burne, J. F. & Raff, M. C. Large-scale normal cell death in the developing rat kidney and its reduction by epidermal growth factor. Development 118, 777–784 (1993).
Meier, P., Finch, A. & Evan, G. Apoptosis in development. Nature 407, 796–801 (2001).
Chautan, M., Chazal, G., Cecconi, F., Gruss, P. & Golstein, P. Interdigital cell death can occur through a necrotic and caspase-independent pathway. Curr. Biol. 9, 967–970 (1999).
Jacobson, M. D., Weil, M. & Raff, M. C. Programmed cell death in animal development. Cell 88, 347–354 (1997).
Mayor, F. & Cuezva, J. M. Hormonal and metabolic changes in the perinatal period. Biol. Neonate 48, 185–196 (1985).
Parham, P. Intolerable secretion in tolerant transgenice mice. Nature 333, 500–503 (1988).
Li, K. et al. Cytochrome c deficiency causes embryonic lethality and attenuates stress-induced apoptosis. Cell 101, 389–399 (2000).
Cecconi, F., Alvarez-Bolado, G., Meyer, B. I., Roth, K. A. & Gruss, P. Apaf1 (CED-4 homolog) regulates programmed cell death in mammalian development. Cell 94, 727–737 (1998).
Yoshida, H. et al. Apaf1 is required for mitochondrial pathways of apoptosis and brain development. Cell 94, 739–750 (1998).
Hakem, R. et al. Differential requirement for caspase 9 in apoptotic pathways in vivo. Cell 94, 339–352 (1998).
Kuida, K. et al. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell 94, 325–337 (1998).
Nakagawa, T. et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-β. Nature 403, 98–103 (2000).
Rao, R. V. et al. Coupling endoplasmic reticulum stress to the cell death program: mechanism of caspase activation. J. Biol. Chem. (in the press).
Nakagawa, T. & Yuan, J. Cross-talk between two cysteine protease families. Activation of caspase 12 by calpain in apoptosis. J. Cell Biol. 150, 887–894 (2000).
Yoneda, T. et al. Activation of caspase-12, an endoplastic reticulum (ER) resident caspase, through tumor necrosis factor recepto-associated factor 2-dependent mechanism in response to the ER stress. J. Biol. Chem. 276, 13935–13940 (2001).
Wegmann, D. R. The immune response to islets in experimental diabetes and insulin-dependent diabetes mellitus. Curr. Opin. Immunol. 8, 860–864 (1996).
Benoist, C. & Mathis, D. Autoimmunity provoked by infection: how good is the case for T cell epitope mimicry? Nature Immunol. 2, 797–801 (2001).
Huang, F.-P. et al. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J. Exp. Med. 191, 435–443 (2000).
Wong, F. S., Visintin, I., Wen, L., Flavell, R. A. & Janeway, C. A. CD8 T cell clones from young nonobese diabetic (NOD) islets can transfer rapid onset of diabetes in NOD mice in the absence of CD4 cells. J. Exp. Med. 183, 67–76 (1996).
Graser, R. T. et al. Identification of a CD8 T cell that can independently mediate autoimmune diabetes development in the complete absence of CD4 T cell helper functions. J. Immunol. 164, 3913–3918 (2000).
Itoh, N. et al. Mononuclear cell infiltration and its relation to the expression of major histocompatibility complex antigens and adhesion moleucles in pancreas biospy speciments from newly diagnosed insulin-dependent diabetes mellitus patients. J. Clin. Invest. 92, 2313–2322 (1993).
Kurrer, M. O., Pakala, S. V., Hanson, H. L. & Katz, J. D. β cell apoptosis in T cell-mediated autoimmune diabetes. Proc. Natl Acad. Sci. USA 94, 213–218 (1997).
Serreze, D. V. et al. Initiation of autoimmune diabetes in NOD/Lt mice is MHC class I-dependent. J. Immunol. 158, 3978–3986 (1997).
Sarukhan, A., Lechner, O. & von Boehmer, H. Autoimmune insulitis and diabetes in the absence of antigen-specific contact between T cells and islet β-cells. Eur. J. Immunol. 29, 3410–3416 (1999).
Thomas, H. E. & Kay, T. W. H. Beta cell destruction in the development of autoimmune diabetes in the non-obese diabetic (NOD) mouse. Diabetes Metab. Res. Rev. 16, 251–261 (2000).
O'Brien, B. A., Harmon, B. V., Cameron, D. P. & Allan, D. J. Apoptosis is the mode of beta-cell death responsible for the development of IDDM in the nonobese diabetic (NOD) mouse. Diabetes 46, 750–757 (1997).
Kagi, D. et al. Reduced incidence and delayed onset of diabetes in perforin-deficient nonobese diabetic mice. J. Exp. Med. 186, 989–997 (1997).
Amrani, A. et al. Perforin-independent β-cell destruction by diabetogenic CD8+ T lymphocytes in transgenic nonobese diabetic mice. J. Clin. Invest. 103, 1201–1209 (1999).
Kagi, D., Odermatt, B., Ohashi, P. S., Zinkernagel, R. M. & Hengartner, H. Development of insulitis without diabetes in transgenic mice lacking perforin-dependent cytotoxicity. J. Exp. Med. 183, 2143–2152 (1996).
Seewaldt, S. et al. Virus-induced autoimmune diabetes: most beta-cells die through inflammatory cytokines and not perforin from autoreactive (anti-viral) cytotoxic T-lymphocytes. Diabetes 49, 1801–1809 (2000).
Kreuwel, H. T. et al. Comparing the relative role of perforin/granzyme versus Fas/Fas ligand cytotoxic pathways in CD8+ T cell-mediated insulin-dependent diabetes mellitus. J. Immunol. 163, 4335–4341 (1999).
Badovinac, V. P., Tvinnereim, A. R. & Harty, J. T. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-gamma. Science 290, 1354–1358 (2000).
Krammer, P. H. CD95's deadly mission in the immune system. Nature 407, 789–795 (2000).
Chervonsky, A. V. et al. The role of Fas in autoimmune diabetes. Cell 89, 17–24 (1997).
Thomas, H. E., Darwiche, R., Corbett, J. A. & Kay, T. W. H. Evidence that β cell death in the nonobese diabetic mouse is Fas independent. J. Immunol. 163, 1562–1569 (1999).
Loweth, A. C., Williams, G. T., James, R. F. L., Scarpello, J. H. B. & Morgan, N. G. Human islets of Langerhans express Fas ligand and undergo apoptosis in response to interleukin-1β and Fas ligation. Diabetes 47, 727–732 (1998).
Itoh, N. et al. Requirement of Fas for the development of autoimmune diabetes in nonobese diabetic mice. J. Exp. Med. 186, 613–618 (1997).
Allison, J. & Strasser, A. Mechanisms of beta cell death in diabetes: a minor role for CD95. Proc. Natl Acad. Sci. USA 95, 13818–13822 (1998).
Kim, Y. H. et al. Apoptosis of pancreatic beta-cells detected in accelerated diabetes of NOD mice: no role of Fas-Fas ligand interaction in autoimmune diabetes. Eur. J. Immunol. 29, 455–465 (1999).
Watanabe, D., Suda, T., Hashimoto, H. & Nagata, S. Constitutive activation of the Fas ligand gene in mouse lymphoproliferative disorders. EMBO J. 14, 12–18 (1995).
Kim, S. et al. Inhibition of autoimmune diabetes by Fas ligand: the paradox is solved. J. Immunol. 164, 2931–2936 (2000).
Su, X. et al. Signficant role for Fas in the pathogenesis of autoimmune diabetes. J. Immunol. 164, 2523–2532 (2000).
Herrera, P. L., Harlan, D. M. & Vassalli, P. A mouse CD8 T cell-mediated acute autoimmune diabetes independent of the perforin and Fas cytotoxic pathways: possible role of membrane TNF. Proc. Natl Acad. Sci. USA 97, 279–284 (2000).
Walter, U. et al. Monitoring gene expressino of TNFR family members by β-cells during development of autoimmune diabetes. Eur. J. Immunol. 30, 1224–1232 (2000).
Beutler, B. A. The role of tumor necrosis factor in health and disease. J. Rheumatol. 57, 16–21 (1999).
Mueller, C., Held, W., Imboden, M. A. & Carnaud, C. Accelerated β-cell destruction in adoptively transferred autoimmune diabetes correlates with an increased expression of the genes coding for TNF-α and granzyme A in the intra-islet infiltrates. Diabetes 44, 112–117 (1995).
Kaneto, H. et al. Apoptotic cell death triggered by nitric oxide in pancreatic β-cells. Diabetes 44, 733–738 (1995).
Mandrup-Poulsen, T., Bendtzen, K., Dinarello, C. A. & Nerup, J. Human tumor necrosis factor potentiates human interleukin 1-mediated rat pancreatic beta-cell cytotoxicity. J. Immunol. 139, 4077–4082 (1987).
Yang, X.-D. et al. Effect of tumor necrosis factor α on insulin-dependent diabetes mellitus in NOD mice. I. The early development of autoimmunity and the diabetogenic process. J. Exp. Med. 180, 995–1004 (1994).
Kagi, D. et al. TNF receptor 1-dependent β cell toxicity as an effector pathway in autoimmune diabetes. J. Immunol. 162, 4598–4605 (1999).
Pakala, S. V., Chivetta, M., Kelly, C. B. & Katz, J. D. In autoimmune diabetes the transition from benign to pernicious insulitis requires an islet cell response to tumor necrosis factor α. J. Exp. Med. 189, 1053–1062 (1999).
Corbett, J. A. & McDaniel, M. L. Intraislet release of interleukin 1 inhibits β cell function by inducing β cell expression of inducible nitric oxide synthase. J. Exp. Med. 181, 559–568 (1995).
Kroncke, K. D., Funda, J., Berschick, B., Kolb, H. & Kolb-Bachofen, V. Macrophage cytotoxicity towards isolated rat islet cells: neither lysis nor its protection by nicotinamide are beta-cell specific. Diabetologia 34, 232–238 (1991).
Corbett, J. A., Lancaster, J. R. Jr, weetland, M. A. & McDaniel, M. L. Interleukin-1β-induced formation of EPR-detectable iron-nitrosyl complexes in islets of Langerhans. J. Biol. Chem. 266, 21351–21354 (1991).
Corbett, J. A. et al. Nitric oxide production in islets from nonobese diabetic mice: aminoguanidine-sensitive and -resistant stages in the immunological diabetic process. Proc. Natl Acad. Sci. USA 90, 8992–8995 (1993).
Rothe, H., Burkart, V., Faust, A. & Kolb, H. Interleukin-12 gene expression is associated with rapid development of diabetes mellitus in non-obese diabetic mice. Diabetologia 39, 119–122 (1996).
Rabinovitch, A. et al. Human pancreatic islet beta-cell destruction by cytokines is independent of nitric oxide production. J. Clin. Endocrinol. Metab. 79, 1058–1062 (1994).
Stassi, G. et al. Nitric oxide primes pancreatic beta cells for Fas-mediated destruction in insulin-dependent diabetes mellitus. J. Exp. Med. 186, 1193–1200 (1997).
Delaney, C. A. et al. Sensitivity of human pancreatic islets to peroxynitrite-induced cell dysfunction and death. FEBS Lett. 394, 300–306 (1996).
Rabinovitch, A., Suarez, W. L., Thomas, P. D., Strynadka, K. & Simpson, I. Cytotoxic effects of cytokines on rat islets: evidence for involvement of free radicals and lipid peroxidation. Diabetologia 35, 409–413 (1992).
Lortz, S. et al. Protection of insulin-producing RINm5F cells against cytokine-mediated toxicity through overexpression of antioxidant enzymes. Diabetes 49, 1123–1130 (2000).
Hohmeier, H. E., Thigpen, A., Tran, W., Davis, R. & Newgard, C. B. Stable expression of manganese superoxide dismutase (MnSOD) in insulinoma cells prevents IL-1β-induced cytotoxicity and reduces nitric oxide production. J. Clin. Invest. 101, 1811–1820 (1998).
Hotta, M. et al. Pancreatic β cell-specific expression of thioredoxin, an antioxidative and antiapoptotic protein, prevents autoimmune and streptozotocin-induced diabetes. J. Exp. Med. 188, 1445–1451 (1998).
Mathews, C. E., Graser, R. T., Savinov, A., Serreze, D. V. & Leither, E. H. Unusual resistance of ALR/Lt mouse beta cells to autoimmune destruction: role for beta cell-expressed resistance determinants. Proc. Natl Acad. Sci. USA 98, 235–240 (2001).
Allison, J. et al. Transgenic overexpression of human Bcl-2 in islet β cells inhibits apoptosis but does not prevent autoimmune destruction. Int. Immunol. 12, 9–17 (2000).
Eizirik, D. L. et al. Major species differences between humans and rodents in the susceptibility to pancreatic β-cell injury. Proc. Natl Acad. Sci. USA 91, 9253–9256 (1994).
Klaus, G. G., Pepys, M. B., Kitajima, K. & Askonas, B. A. Activation of mouse complement by different classes of mouse antibody. Immunology 38, 687–695 (1979).
Bach, J. F. Insulin-dependent diabetes mellitus as an autoimmune disease. Endocr. Rev. 15, 516–542 (1994).
Taylor, S. I. Deconstructing type 2 diabetes. Cell 97, 9–12 (1999).
Shimabukuro, M., Ohneda, M., Lee, Y. & Unger, R. H. Islet amyloid, increased A-cells, reduced B-cells and exocrine fibrosis: quantitative changes in the pancreas in type 2 diabetes. Diabetes Res. 9, 151–159 (1988).
Sempoux, C., Guiot, Y., Dubois, D., Moulin, P. & Rahier, J. Human type 2 diabetes: morphological evidence for abnormal β-cell function. Diabetes 50, S172–S177 (2001).
Guiot, Y., Sempoux, C., Moulin, P. & Rahier, J. No decrease of the β-cell mass in type 2 diabetic patients. Diabetes 50, S188 (2001).
Federici, M. et al. High glucose causes apoptosis in cultured human pancreatic islets of Langerhans. Diabetes 50, 1290–1301 (2001).
Bar-On, H., Ben-Sasson, R., Ziv, E., Arar, N. & Shafrir, E. Irreversibility of nutritionally induced NIDDM in Psammomys obesus is related to beta-cell apoptosis. Pancreas 18, 259–265 (1999).
Donath, M. Y., Gorss, D. J., Cerasi, E. & Kaiser, N. Hyperglycemia-induced β-cell apoptosis in pancreatic islets of psammomys obesus during development of diabetes. Diabetes 48, 738–744 (1999).
Leibowitz, G. et al. β-cell glucotoxicity in the psammomys obesus model of type 2 diabetes. Diabetes 50, S113–S117 (2001).
Shimabukuro, M., Zhou, Y. T., Levi, M. & Unger, R. H. Fatty acid-induced beta cell apoptosis: a link between obesity and diabetes. Proc. Natl Acad. Sci. USA 95, 2498–2502 (1998).
Efanova, I. B. et al. Glucose and tolbutamide induce apoptosis in pancreatic beta-cells: a process dependent on intracellular Ca2+ concentration. J. Biol. Chem. 273, 33501–33507 (1998).
Withers, D. J. et al. Irs-2 coordinates Igf-1 receptor-mediated β-cell development and peripheral insulin signalling. Nature Genet. 23, 32–40 (1999).
Harding, H. P. et al. Diabetes mellitus and exocrine pancreatic dysfunction in perk−/− mice reveals a role for translational control in secretory cell survival. Mol. Cell 7, 1153–1163 (2001).
Gilligan, M. et al. Glucose stimulates the activity of the guanine nucleotide-exchange factor eIF-2B in isolated rat islets of Langerhans. J. Biol. Chem. 271, 2121–2125 (1996).
Delepine, M. et al. EIF2AK3, encoding translation initiation factor 2-α kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nature Genet. 25, 406–409 (2000).
Guerder, S., Eynon, E. E. & Flavell, R. A. Autoimmunity without diabetes in transgenic mice expressing β cell-specific CD86, but not CD80: parameters that trigger progression to diabetes. J. Immunol. 161, 2128–2140 (1998).
Katz, J. D., Wang, B., Haskins, K., Benoist, C. & Mathis, D. Following a diabetogenic T cell from genesis through pathogenesis. Cell 74, 1089–1100 (1993).
Ohashi, P. S. et al. Ablation of “tolerance” and induction of diabetes by virus infection in viral antigen transgenic mice. Cell 65, 305–317 (1991).
Sarvetnick, N., Liggitt, D., Pitts, S. L., Hansen, S. E. & Stewart, T. A. Insulin-dependent diabetes mellitus induced in transgenic mice by ectopic expression of class II MHC and interferon-gamma. Cell 52, 773–782 (1988).
Acknowledgements
We apologize to the many investigators whose work we could not cite due to a limit on the number of references. We thank C. R. Kahn for his insights into type 2 diabetes. Our work on β-cell death is funded by a research grant from the Juvenile Diabetes Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mathis, D., Vence, L. & Benoist, C. β-Cell death during progression to diabetes. Nature 414, 792–798 (2001). https://doi.org/10.1038/414792a
Issue Date:
DOI: https://doi.org/10.1038/414792a
This article is cited by
-
Association of CIITA (rs8048002) and CLEC2D (rs2114870) gene variants and type 1 diabetes mellitus
Journal of Diabetes & Metabolic Disorders (2024)
-
Integrated biomarker profiling of the metabolome associated with type 2 diabetes mellitus among Tibetan in China
Diabetology & Metabolic Syndrome (2023)
-
Discovery of IHMT-MST1-39 as a novel MST1 kinase inhibitor and AMPK activator for the treatment of diabetes mellitus
Signal Transduction and Targeted Therapy (2023)
-
Dysfunctions, molecular mechanisms, and therapeutic strategies of pancreatic β-cells in diabetes
Apoptosis (2023)
-
Integrated glycomics and genetics analyses reveal a potential role for N-glycosylation of plasma proteins and IgGs, as well as the complement system, in the development of type 1 diabetes
Diabetologia (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.