Abstract
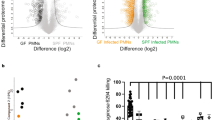
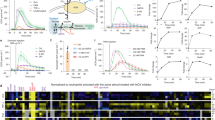
In response to environmental stimuli, leukocyte membrane remodelling generates biologically active lipids that can serve as both intra- and extracellular mediators1. There are several classes of lipids that can mediate inflammatory reactions.1 We report here on a new intracellular lipid signal that regulates oxygen-radical formation in neutrophils, a key response in microbial killing, inflammation and tissue injury. Screening of neutrophil-derived extracts rich in phosphorylated, non-saponifiable lipids revealed a potent inhibitor of superoxide anion (O2−) production. Structural analysis of biologically active fractions gave four major phosphorylated lipids: most abundant was presqualene diphosphate (PSDP). Upon activation of neutrophil receptors, PSDP and its monophosphate form, presqualene monophosphate (PSMP), undergo rapid remodelling. At submicromolar concentrations, PSDP but not PSMP inhibit O2− production by human neutrophil cell-free oxidase preparations. We prepared PSDP and PSMP by total organic synthesis and matched both the physical properties and biological activity of the neutrophil-derived compounds. Our results indicate that PSDP, a recognized intermediate of cholesterol biosynthesis2, is present in immune effector cells and is a potent regulator of the cellular response in host defence.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Serhan, C. N., Haeggström, J. Z. & Leslie, C. C. Lipid mediator networks in cell signaling: Update and impact of cytokines. FASEB J. 10, 1147 –1158 (1996).
Jarstfer, M. B., Blagg, B. S. J., Rogers, D. H. & Poulter, C. D. Biosynthesis of squalene. Evidence for a tertiary cyclopropylcarbinyl cationic intermediate in the rearrangement of presqualene diphosphate to squalene. J. Am. Chem. Soc. 118, 13089– 13090 (1996).
Weissmann, G., Smolen, J. E. & Korchak, H. M. Release of inflammatory mediators from stimulated neutrophils. N. Engl. J. Med. 303, 27– 34 (1980).
Adair, W. L. & Keller, R. K. Isolation and assay of dolichol and dolichyl phosphate. Meth. Enzymol. 111, 201–215 (1985).
Van Dessel, G. A. F., Lagrou, A. R., Hilderson, H. J. J. & Dierick, W. S. H. in CRC Handbook of Chromatography (eds Mukherjee, K. D., Weber, N. & Sherma, J.) 321–337 (CRC Press, Boca Raton, (1993)).
McPhail, L. C., Shirley, P. S., Clayton, C. C. & Synderman, R. Activation of the respiratory burst enzyme from human neutrophils in a cell-free system. Evidence for a soluble cofactor. J. Clin. Invest. 75, 1735–1739 (1985).
Agwu, D. E., McPhail, L. C., Sozzani, S., Bass, D. A. & McCall, C. E. Phosphatidic acid as a second messenger in human polymorphonuclear keukocytes. Effects on activation of NADPH oxidase. J. Clin. Invest. 88, 531– 539 (1991).
Bokoch, G. M. & Knaus, U. G. Ras-related GTP-binding proteins and leukocyte signal transduction. Curr. Opin. Hematol. 1, 53–60 (1994).
Farnsworth, C. C., Gelb, M. H. & Glomset, J. A. Identification of geranylgeranyl-modified proteins in HeLa cells. Science 247, 320– 322 (1990).
Popják, G., Edmond, J., Clifford, K. & Williams, V. Biosynthesis and structure of a new intermediate between farnesyl pyrophosphate and squalene. J. Biol. Chem. 244, 1897– 1918 (1969).
Epstein, W. W. & Rilling, H. C. Studies on the mechanism of squalene biosynthesis. The structure of presqualene pyrophosphate. J. Biol. Chem. 245, 4597– 4605 (1970).
Goldstein, J. L. & Brown, M. S. Regulation of the mevalonate pathway. Nature 343, 425– 430 (1990).
Corey, E. J. & Volante, R. P. Application of unreactive analogs of terpenoid pyrophosphates to studies of multistep biosynthesis. Demonstration that “presqualene pyrophosphate” is an essential intermediate on the path to squalene. J. Am. Chem. Soc. 98, 1291–1293 (1976).
Mookhtiar, K. A., Kalinowski, S. S., Khang, D. & Poulter, C. D. Yeast squalene synthase. A mechanism for addition of substrates and activation by NADPH. J. Biol. Chem. 269, 11201– 11207 (1994).
Shechter, I., Fogelman, A. M. & Popjak, G. Adeficiency of mixed function oxidase activities in the cholesterol biosynthetic pathway of human granulocytes. J. Lipid Res. 21, 277–283 ( 1980).
Philips, M. R. et al. Carboxyl methylation of ras-related proteins during signal transduction in neutrophils. Science 259, 977–980 (1992).
Baggiolini, M., Boulay, F., Badwey, J. A. & Curnutte, J. T. Activation of neutrophil leukocytes: chemoattractant receptors and respiratory burst. FASEB J. 7, 1004– 1010 (1993).
Gomez-Cambronero, J. & Sha'afi, R. I. in Cell–Cell Interactions in the Release of Inflammatory Mediators (eds Wong, P. Y.-K. & Serhan, C. N.) 35–71 (Plenum, New York, (1991)).
Scheer, A. & Gierschik, P. S-prenylated cysteine analogues inhibit receptor-mediated G protein activation in native human granulocyte and reconstituted bovine retinal rod outer segment membranes. Biochemistry 34, 4952–4961 (1995).
Simons, K. & Ikonen, E. Functional rafts in cell membranes. Nature 387, 569–572 (1997).
Rogers, D. H., Yi, E. C. & Poulter, C. D. Enantioselective synthesis of (+)-presqualene diphosphate. J. Org. Chem. 60, 941– 945 (1995).
Quinn, M. T. Low-molecular-weight GTP-binding proteins and leukocyte signal transduction. J. Leuk. Biol. 58, 263– 276 (1995).
Maddox, J. F. & Serhan, C. N. Lipoxin A4and B 4are potent stimuli for human monocyte migration and adhesion: selective inactivation by dehydrogenation and reduction. J. Exp. Med. 183, 137–146 (1996).
Chen, P. S., Toribara, T. Y. & Warner, H. Microdetermination of phosphorus. Analyt. Chem. 28, 1756–1758 ( 1956).
Tou, J. & Dola, T. Leukotriene B4stimulation of an early elevation of phosphatidic acid mass in human neutrophils. Lipids 30, 373–381 ( 1995).
Bromberg, Y. & Pick, E. Unsaturated fatty acids stimulate NADPH-dependent superoxide production by cell-free system derived from macrophages. Cell. Immunol. 88, 213–221 (1984).
Acknowledgements
This work was supported in part by the NIH (GM & NIDDK) and a basic discovery research grant from ONO Pharmaceuticals to C.N.S. and the NIH (GM) to N.A.P. B.D.L. was supported in part by a Paul Dudley White postdoctoral fellowship of the Massachusetts Affiliate, American Heart Association. We thank Valery V. Fokin for help with the synthesis and the NMR analyses of PSDP and PSMP.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Rights and permissions
About this article
Cite this article
Levy, B., Petasis, N. & Serhan, C. Polyisoprenyl phosphates in intracellular signalling. Nature 389, 985–990 (1997). https://doi.org/10.1038/40180
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/40180
This article is cited by
-
Lipid-Derived Mediators are Pivotal to Leukocyte and Lung Cell Responses in Sepsis and ARDS
Cell Biochemistry and Biophysics (2021)
-
Novel polyisoprenyl phosphates block phospholipase D and human neutrophil activation in vitro and murine peritoneal inflammation in vivo
British Journal of Pharmacology (2005)
-
Cross-coupling reaction of cyclopropylboronic acids with aryl ω-halo-oxo-perfluoroalkylsulfonates
Chinese Science Bulletin (2001)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.