Abstract
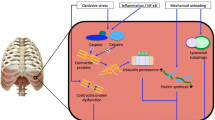
It became evident in the past 12 years that venitlatory muscle contractile performance is significantly impaired during the course of septic shock. In animal models of septic shock, depression of ventilatory muscle contractile performance has been shown to cause hypercapneic ventilatory failure and respiratory arrest. Failure of ventilatory muscle contractility in septic shock has never been attributed to a single factor, but two groups of factors are likely to be involved: (a) increased ventilatory muscle metabolic demands due to augmentation of ventilation, hypoxemia and increased pulmonary impedance; and (b) specific cellular, metabolic, immune and hemodynamic defects which interfere with several processes necessary for normal force generation. These defects are mediated by complex interactions between several local and systematic mediator such a bacterial endotoxin, proinflammatory cytokines, prostaglandins, platelet activating factor, reactive oxygen species and nitric oxide. This is a summary of how these interactions are likely to interfere with ventilatory muscle contractile performance in septic shock with particular emphasis on the newly described role of nitric oxide.
Similar content being viewed by others
References
Burke JF, Pontopiddan H, Welch CE: High output respiratory failure. Ann Sung 158: 581–594, 1963
Pontopiddan H, Geffin B, Laver MB: Respiratory failure in septic shock. In: SG Hershey, LRM Del Guerico (eds). Septic Shock in Man. (ed. First edition). Boston, Little, Brown and Comp. 1968, pp 37–45
Cohen CA, Zagelbaum G, Gross D, Roussos C, Macklem PT: Clinical manifestation of inspiratory muscle fatigue. Am J Med 73: 308–316, 1982
Friman G: Effects of acute infectious disease on isometric strength. Scand J Clin Lab Invest 37: 303–308, 1977
Ruff RL, Secrist D: Inhibitors of prostaglandin synthesis or cathespin B prevent muscle wasting due to sepsis in the rat. J Clin Invest 73: 1483–1486, 1984
Murphy TD, Gibson RL, Standaert TA, Woodrum DE: Diaphragmatic failure during group B streptococcal sepsis in piglets: the role of thromboxane A2. J Appl Physiol 78: 491–498, 1995
Leon A, Boczkowski J, Dureuil B, Desmonts JM, Aubier M: Effects of endotoxic shock on diaphragmatic function in mechanically ventilated rats. J Appl Physiol 72: 1466–1472, 1992
Shindoh C, Hida W, Ohkawara Y, Yamauchi K, Ohno I, Takishima T, Shirato K: TNF-αmRNA expression in diaphragm muscle after endotoxin administration. Am J Resp Crit Care Med 152: 1690–1696, 1995
Boczkowski J, Dureuil B, Pareinte R, Aubier M: Preventive effects of indomethacin n diaphragmatic contractile alterations in endotoxemic rats. Am Rev Respir Dis 142: 193–198, 1990
Shindoh C, DiMarco A, Nethery D, Supinski G: Effect of PEG-superoxide dismutase on the diaphragmatic response to endotoxin. Am Rev Respir Dis 145: 1350–1354, 1992
Supinski G, Nerthery D, DiMarco A: Effect of free radical scavengers on endotoxin-induced respiratory muscle dysfunction. Am Rev Respir Dis 148: 13318–1324, 1993
Boczkowski J, Dureuil B, Brauger C, Pavlovic D, Murciano D, Pariente R, Aubier M: Effect of sepsis on diaphragmatic function in rats. Am Rev Respir Dis 138: 260–265, 1988
Hussain SNA, Simkus G, Roussos C: Respiratory muscle fatigue: A cause of ventilatory failure in septic shock. J Appl Physiol 58: 2033–2040, 1985
Diaz PT, Julian MW, Wewers MD, Clanton TL: Tumor necrosis factor and endotoxin do not directly affect in-vitro diaphragm function. Am Rev Respir Dis 148: 281–287, 1993
Wilcox PG, Wakai Y, Walley KR, Cooper DJ, Road, J: Tumor necrosis factor ??decreases in vivo diaphragm contractility in dogs. Am J Resp Crit Care Med 150: 1368–1373, 1994
Hussain SNA, Roussos C, Magder S: Autoregulation of diaphragmatic blood flow in dogs. J Appl Physiol 64: 329–336, 1968
Hussain SNA, Graham R, Rutledge F, Roussos C: Respiratory muscle engergetics during endotoxic shock in dogs. J Appl Physiol 60: 486–493, 1986
Bond RF, Scott CG, Krech LH, Bond CH: Systematic and local effects of endotoxin on canine gracilis muscle vascular conductance. Am J Physiol 258: H1498–H1506, 1990
Auclair MC, Svinareff P, Schmitt H: Vascular α-adrenoreceptor blockade of E. coli endotoxin in the rat. Eur J Pharamcol 127: 121–124, 1986
Spitzer JA, Turco ER, Deaciuc IV: Pertubation of transmembrane signaling mechanisms in acute and chronic endotoxemia. In: Anonymous First Vienna Shock Form, Part A: Pathophysiological role of Mediators and Mediator Inhibitors in Shock. New York, 1987, pp 401–418
Beasley D, Cohen RA, Levinsky NG: Interleukin-1 inhibits contraction of vascular smooth muscle. J Clin Invest 83: 331–335, 1989
Kilbourn RG, Griffith OW: Overproductin of nitric oxide in cytokinemediated and septic shock. J Natl Cancer Inst 84: 827–831, 1992
Julou-Schaeffer G, Gray GA, Fleming I: Loss of vascular responsiveness induced by endotoxin involves L-arginine pathway. Am J Physiol 259: H1038–H1043, 1990
Boczkowski J, Vicaut E, Aubier M: In vivo effects of Echerichia coli endotoxemia on diaphragmatic microcirculation in rats. J Appl Physiol 72: 2219–2224, 1992
Kim WS, Ward ME, Hussain SNA: Pathological O2 supply-dependence of diaphragmatic and sytematic O2 uptake during endotoxemia. J Appl Physiol 77: 1093–1100, 1994
Holecek M, Skopec F, Sprong L, Pecka M: Protein metabolism in specific tissues of endotoxin-treated rats: effect of nutritional status. Physiol Res 44(6): 399–406, 1995
Zamir O, Hasselgren PO, O'Brien W, Thompson RC, Fischer JE: Muscle protein breakdown during endotomemia in rats and after treatment with interlukin-1 receptor antagonists (IL-1a). Ann Surg 216(3): 381–385, 1992
Mela L, Miller LD, Bacalzo LV, Olofsson K, White RR: Role of intracellular variations of lysosomal enzyme activity and oxygen tension in mitochondrial impairments in endotoxemia and hemorrhage in the rat. Ann Surg 178: 727–735, 1973
Parker CJ, Sardesai VM: The effects of E. coli endotoxin on cardiac and skeletal muscle myofibrillar ATPase. Biochem Med 8: 11–17, 1973
Hess ML, Soulsby ME, Davies JA, Briggs FN: The influence of venous return on cardiac mechanical and sarcoplasmic reticulum function durin endotoxemia. Circ Shock 4: 143–152, 1977
Marletta MA, Yoon PS, Lyengar R, Leaf CD, Wishnok JS: Macrophage oxidation of L-arginine to nitrite and nitrate. Biochem 24: 8706–8711, 1988
Sakuma I, Stuehr DJ, Gross SS, Nathan CF, Levi R: Identification of arginine as a precursor or endothelium-derived relaxing factor. Proc Natl Acad Sci 85: 8664–8667, 1988
Valance P, Collier J, Moncada S: Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet 2: 977–1000, 1989
Ward ME, Magder S, Hussain SNA: The role of endothelium-derived relaxing factor in reactive hyperemia of the canine diaphragm. J Appl Physiol 74: 1606–1612, 1993
Persson MG, Gustafsson LE, Wiklund NP, Hedqvist P, Moncada S: Endogenous nitric oxide as a modulator of rabbit skeletal muscle microcirculation. Br J Pharmacol 10: 463–466, 1990
Kelm M, Schrader J: Nitric oxide release from the isolated guinea pig heart. Eur J Pharmacol 155: 317–321, 1988
Ward ME, Magder SA, Hussain SNA: Role of endothelium-derived relaxing factor in reactive hyperaemia in the diaphragm. J Appl Physiol 74(4): 1606–1612, 1993
Ward ME, Hussain SNA: Diaphragmatic pressure-flow relationship during hemorrhagic shock: Role of nitric oxide. J Appl Physiol 77: 2244–2249, 1994
Hussain SNA, Stewart DJ, Ludemann JP, Magder S: The role of endothelium-derived relaxing factors (EDRF) in active hyperaemia of the canine diaphragm. J Appl Physiol 72: 2393–2401, 1992
Chang HY, Ward ME, Hussain SNA: Regulationof diaphragmatic oxygen uptake by endothelium-derived relaxing factor (EDRF). Am J Physiol 265: H123–H130, 1993
Nakane M, Schmidt HHHW, Pollock JS, Forstermann U, Murad F: Cloned human brain nitric oxide synthase is highly expressed in skeletal muscle. Federation of European Biomedical Societies 316: 175–180, 1993
Kobzik L, Reid MB, Bredt DS, Stamler JS: Nitric oxide in skeletal muscle. Nature 372: 546–548, 1994
Balon TW, Nadler JL: Nitric oxide release is present from incubated skeletal muscle preparations. J Appl Physiol 77: 2519–2521, 1994
Kobzik L, Stringer B, Balligand JL, Reid MB, Stamler JS.: Endothelial type nitric oxide synthase in skeletal muscle fibers: Mitochondrial relationship. Biochem Biophys Res Commun 211: 375–381, 1995
Stuehr DJ, Marletta M: Further studies on murine macrphage synthesis of nitrite and nitrate. In: H Bartsch, IK O'Neill, R Schulte-Hermann (eds). Relevance of N-nitroso Compounds to Human Cancer: Exposures and Mechanisms. Lyon, IARC Scientific Publication, 1987, pp 335–339
Lamas S, Michel T, Brenner BM, Marsden PA: Nitric oxide synthase in endothelial cells – evidence for a pathway inducible by TNF-±. Am J Physiol 261: C634–C641, 1991
Beasley RE, Cohen RA, Levinsky NG: Endotoxin inhibits contraction of vascular smooth muscle in vitro. Am J Physiol 258: H187–H1192, 1990
Liu S, Adcock IM, Old RW, Barnes PJ, Evans TW: Lipopolysaccharide treatment in vivo induces widespread tissue expression of inducible nitric oxide synthase mRNA. Biochem Biophys Res Comm 196: 1208–1213, 1993
Preiser JC, Lejeune P, Roman A, Carlier E, De Backer D, Leeman M, Kahn RJ, Vincent JL: Methylene blue administration in septic shock: A clinical trial. New Horizons 23: 259–264, 1995
Szabo CS, Mitchell JA, Thimerman C, Vane JR: Nitric oxide-induced hyporeactivity to noradrenaline precedes the induction of nitric oxide synthase in endotoxin shock. Br J Pharmacol 108: 786–792, 1993
Salter M, Knowles RG, Moncada S: Widespread tissue distribution, species distribution and changes in activity of Ca2+ and Ca2+ independent nitric oxide synthases. FEBS Lett 291: 145–149, 1991
Thiemermann C, Vane JR: Inhibition of nitric oxide synthesis reduces the hypotension induced by bacterial lippolyaccharides in the rat in vivo. Eur J Pharmacol 182: 591–595, 1990
Hussain SNA: Role of nitric oxide in endotoxin-induced metabolic and vascular dysregulation of the canine diaphragm. Am J Respr Crit Care Med 152: 683–689, 1995
Hussain SNA, El-Dawiri Q, Sakkal D, Hattori R, Guo Y: Expression of inducible nitric oxide synthase and GTP cyclohydrolase I in the ventilatory and limb muscles during endotoxemia. Am J Respir Cell Mol Biol 17: 173–180, 1997
Cleeter MWJ, Cooper JM, Darley-Usmar VM, Moncada S, Schapira AHV: Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. FEBS Lett 345: 50–54 (Abstract)
Torres, Darley-Usmar VM, Wilson MT: Inhibition of cytochrome c oxidase in turnover by nitric oxide: Mechanism and impliations for control of respiration. Biochem J 312: 1002+1009, 1995
Crow JP, Beckman JS: The role of peroxynitrite in nitric oxide-mediated toxicity. Curr Topics Microbio Immunol 196: 57–73, 1995
Radi R, Rodriguez M, Castro L, Telleri R: Inhibition of mitochondrial electron transport by peroxynitrite. Arch Bioch Bioph 308: 89–95, 1994
Hershey JC, Bond RF: Endotoxin induces metabolic dysregulation of vascular tone. Am J Physiol 265: H108–113, 1993
Molina Y, Vedia L, McDonald B, Reep B, Brune B, Di Silvo M, Billier TR, Lapetina EG: Nitric oxide-induced s-nitrosylation of glyceraldehyde-3-phospate dehydrogenase inhibits enzymatic activity and increases endogenous ADP-ribosylation. J Biol Chem 267: 23929–24932, 1992
Meszaros LG, Minarovic I, Zahradnikova A: Inhibition of the skeletal muscle ryanodine receptor calcium release by nitric oxide. FEBS Lett 380: 49–52, 1996
Brotto MA, Nosek TM: Hydrogen peroxide disrupts Ca2+ release from the sarcoplasmic reticulum of rat skeletal muscle fibers. J Appl Physiol 81(2): 731–737, 1996
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hussain, S.N.A. Repiratory muscle dysfunction in sepsis. Mol Cell Biochem 179, 125–134 (1998). https://doi.org/10.1023/A:1006864021783
Issue Date:
DOI: https://doi.org/10.1023/A:1006864021783