Abstract
Background
As more studies report on patient preferences, techniques are needed to identify, assess and, eventually, synthesize results from a diverse set of methodologies. Data on patient preferences are valuable to decision makers in a variety of ways. Preferences for outcomes can be used to inform decision and cost-effectiveness models, while preferences for treatments can inform patient-centered outcomes research (PCOR) and patient-centered care.
Objectives
This project sought to identify and assess the literature reporting on the treatment preferences of adult patients with type 2 diabetes. In addition to cataloging the preference elicitation methods used, we developed and assessed a novel quality assessment checklist for preference-based studies.
Data sources
PubMed, EMBASE, CINAHL, and EconLit databases were searched to identify studies examining patient preferences for medications for type 2 diabetes studies published since inception of each database.
Study eligibility criteria, participants, and interventions
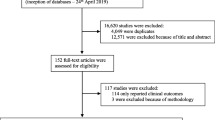
The review protocol specified inclusion of studies reporting diabetes-treatment preferences among adults with type 2 diabetes, using a range of preference measurement methods. Studies were excluded if participants were not patients with type 2 diabetes and if treatments were not pharmacological therapies targeting glycemic control, or if no primary preference information was collected. Two investigators independently reviewed titles, abstracts, and articles sequentially to select studies for data abstraction based on the inclusion and exclusion criteria. Disagreements were resolved by consensus.
Study appraisal and synthesis methods
Data on study country, year, number of respondents, preference elicitation method, number of attributes, subgroup analyses, and funding source were abstracted into standardized tables. A novel checklist (PREFS) was used to assess the data quality and validity across different types of preference studies by assessing the following: purpose of the study; respondent sampling; explanation of preference assessment methods; findings reported for total sample; and significance testing. Each item was scored, and an aggregate score was then calculated (ranging from 0 to 5).
Results
Of the 2,100 unique citations, 61 met the inclusion criteria. The studies used conjoint analysis (n = 10), time trade-off (n = 6), standard gamble (n = 2), contingent valuation (n = 1), other stated preference methods (n = 39), and revealed preferences (n = 5). Sample sizes ranged from 27 to 14,033, with an average of 562 respondents, and two-thirds included a subgroup analysis. Most studies were conducted in one country, predominantly the USA (n = 27), UK (n = 14), Canada (n = 10), and Germany (n = 7), while 14 were conducted in multiple (2–18) countries across two or more countries. There was an increase in the annual rate of studies published over time from the time of the first publication in 1985 (p = < 0.01). Most (n = 52) studies were funded by pharmaceutical or device companies, with government, academic, association, and hospital sources also funding studies. One study met all five of the PREFS criteria and 12 met four; yet four studies met none of the criteria. The average was 27.
Limitations
Currently, preferences reviews are limited by the mixed quality in the reporting of studies, the publication bias inherent in the literature, a lack of guidelines to conduct various methods, and the difficulty of synthesizing results from different studies. Our study is also limited by its focus on English language articles.
Conclusions and implications of key findings
This study provides the first systematic evaluation of the methods used in the broad existing body of research into patient preferences for type 2 diabetes medications and can serve as a primary source of information for decision makers. Future work is necessary to assess the utility of the results of reviews of preference information and to develop best-practice guidelines for the reporting of, and methods of conducting, preference studies and systematic reviews of such studies.
Registration
This systematic review was registered with PROSPERO (registration number CRD42012002285).


Similar content being viewed by others
References
Patient Protection and Affordable Care Act (2010) (USA). H. R. 3590. United States Government Printing Office, USA.
Frosch DL, Moulton BW, Wexler RM, Holmes-Rovner M, Volk RJ, Levin CA. Shared decision making in the United States: policy and implementation activity on multiple fronts. Z Evid Fortbild Qual Gesundhwes. 2011;105(4):305–12.
Bridges JF. Stated preference methods in health care evaluation: an emerging methodological paradigm in health economics. Appl Health Econ Health Policy. 2003;2(4):213–24.
Bridges JF. Future challenges for the economic evaluation of healthcare: patient preferences, risk attitudes and beyond. Pharmacoeconomics. 2005;23(4):317–21.
Bridges JF. Lean systems approaches to health technology assessment: a patient-focused alternative to cost-effectiveness analysis. Pharmacoeconomics. 2006;24(Suppl 2):101–9.
Bridges JFP, Jones C. Patient-based health technology assessment: a vision of the future. Int J Technol Assess Health Care. 2007;23(1):30–5.
Vogt F, Schwappach DL, Bridges JF. Accounting for tastes: a German perspective on the inclusion of patient preferences in healthcare. Pharmacoeconomics. 2006;24(5):419–23.
Bridges JFP, Kinter ET, Kidane L, Heinzen RR, McCormick C. Things are looking up since we started listening to patients: Trends in the application of conjoint analysis in health 1982–2007. Patient. 2008;1(4):273–82.
Patient Centered Outcomes Research Institute. Patient-centered outcomes research (Internet). 2013. http://www.pcori.org/research-we-support/pcor/.
Hurtado MP, Swift EK, Corrigan J. Envisioning the national health care quality report. Washington, D.C.: National Academies Press; 2001.
Baumann MH, Lewis SZ, Gutterman D, American College of Chest Physicians. ACCP evidence-based guideline development: a successful and transparent approach addressing conflict of interest, funding, and patient-centered recommendations. Chest. 2007;132(3):1015–24.
Connor Gorber S, Singh H, Pottie K, Jaramillo A, Tonelli M. Process for guideline development by the reconstituted Canadian task force on preventive health care. CMAJ. 2012;184(14):1575–81.
Qaseem A, Snow V, Owens DK, Shekelle P, Clinical Guidelines Committee of the American College of Physicians. The development of clinical practice guidelines and guidance statements of the American College of Physicians: summary of methods. Ann Intern Med. 2010;153(3):194–9.
Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35(6):1364–79.
Samuelson PA. A note on measurement of utility. Rev Econ Stud. 1937;4(2):155–61.
Bridges J, Onukwugha E, Johnson F, Hauber A. Patient preference methods—a patient centered evaluation paradigm. ISPOR Connect. 2007;13(6):4–7.
Kaplan RM. Health outcome models for policy analysis. Health Psychol. 1989;8(6):723–35.
Torrance GW, Furlong W, Feeny D, Boyle M. Multi-attribute preference functions. Health Utilities Index. Pharmacoeconomics. 1995;7(6):503–20.
Ajzen I. Attitude structure and behavior. In: Pratkanis AR, Breckler SJ, Greenwald AG, editors. Attitude structure and function. Hillsdale: Lawrence Erlbaum Associates; 1989. p. 241–74.
Marshall D, McGregor SE, Currie G. Measuring preferences for colorectal cancer screening: what are the implications for moving forward? Patient. 2010;3(2):79–89.
Gooberman-Hill R. Qualitative approaches to understanding patient preferences. Patient. 2012;5(4):215–23.
Ryan M, Farrar S. Using conjoint analysis to elicit preferences for health care. BMJ. 2000;320(7248):1530–3.
Gafni A. The standard gamble method: what is being measured and how it is interpreted. Health Serv Res. 1994;29(2):207–24.
Torrance GW, Thomas WH, Sackett DL. A utility maximization model for evaluation of health care programs. Health Serv Res. 1972;7(2):118–33.
Diener A, O’Brien B, Gafni A. Health care contingent valuation studies: a review and classification of the literature. Health Econ. 1998;7(4):313–26.
Phillips KA, Johnson FR, Maddala T. Measuring what people value: a comparison of “attitude” and “preference” surveys. Health Serv Res. 2002;37(6):1659–79.
Mark TL, Swait J. Using stated preference and revealed preference modeling to evaluate prescribing decisions. Health Econ. 2004;13(6):563–73.
Sauerland S, Seiler CM. Role of systematic reviews and meta-analysis in evidence-based medicine. World J Surg. 2005;29(5):582–7.
Gilbody SM, Petticrew M. Rational decision-making in mental health: the role of systematic reviews. J Ment Health Policy Econ. 1999;2(3):99–106.
Dennis CL, Chung-Lee L. Postpartum depression help-seeking barriers and maternal treatment preferences: a qualitative systematic review. Birth. 2006;33(4):323–31.
Higginson IJ, Sen-Gupta GJ. Place of care in advanced cancer: a qualitative systematic literature review of patient preferences. J Palliat Med. 2000;3(3):287–300.
Lin OS, Kozarek RA, Gluck M, Jiranek GC, Koch J, Kowdley KV, et al. Preference for colonoscopy versus computerized tomographic colonography: a systematic review and meta-analysis of observational studies. J Gen Intern Med. 2012;27(10):1349–60.
Mazzoni A, Althabe F, Liu NH, Bonotti AM, Gibbons L, Sanchez AJ, et al. Women’s preference for caesarean section: a systematic review and meta-analysis of observational studies. BJOG. 2011;118(4):391–9.
Lubeck DP, Grossfeld GD, Carroll PR. A review of measurement of patient preferences for treatment outcomes after prostate cancer. Urology. 2002;60(3 Suppl 1):72–7.
Morales AM, Casillas M, Turbi C. Patients’ preference in the treatment of erectile dysfunction: a critical review of the literature. Int J Impot Res. 2011;23(1):1–8.
Parker SM, Clayton JM, Hancock K, Walder S, Butow PN, Carrick S, et al. A systematic review of prognostic/end-of-life communication with adults in the advanced stages of a life-limiting illness: patient/caregiver preferences for the content, style, and timing of information. J Pain Symptom Manage. 2007;34(1):81–93.
Say R, Murtagh M, Thomson R. Patients’ preference for involvement in medical decision making: a narrative review. Patient Educ Couns. 2006;60(2):102–14.
van Schaik DJ, Klijn AF, van Hout HP, van Marwijk HW, Beekman AT, de Haan M, et al. Patients’ preferences in the treatment of depressive disorder in primary care. Gen Hosp Psychiatry. 2004;26(3):184–9.
Wilkinson EK, Salisbury C, Bosanquet N, Franks PJ, Kite S, Lorentzon M, et al. Patient and carer preference for, and satisfaction with, specialist models of palliative care: a systematic literature review. Palliat Med. 1999;13(3):197–216.
Gomes B, Calanzani N, Gysels M, Hall S, Higginson IJ. Heterogeneity and changes in preferences for dying at home: a systematic review. BMC Palliat Care. 2013;12(1):7.
Phillips KA, Van Bebber S, Marshall D, Walsh J, Thabane L. A review of studies examining stated preferences for cancer screening. Prev Chronic Dis. 2006;3(3):A75–82.
Krahn M, Naglie G. The next step in guideline development. JAMA. 2008;300(4):436.
Boivin A, Green J, van der Meulen J, Legare F, Nolte E. Why consider patients’ preferences? A discourse analysis of clinical practice guideline developers. Med Care. 2009;47(8):908–15.
Crump RT, Llewellyn-Thomas HA. The importance of measuring strength-of-preference scores for health care options in preference-sensitive care. J Clin Epidemiol. 2012;65(8):887–96.
Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32.
Levac D, Colquhoun H, O’Brien KK. Scoping studies: advancing the methodology. Implement Sci. 2010;5:69–77.
Daudt HM, van Mossel C, Scott SJ. Enhancing the scoping study methodology: a large, inter-professional team’s experience with Arksey and O’Malley’s framework. BMC Med Res Methodol. 2013;13:48–56.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
Ali S, Ronaldson S. Ordinal preference elicitation methods in health economics and health services research: using discrete choice experiments and ranking methods. Br Med Bull. 2012;103(1):21–44.
Oliver RL, Linda G. Effect of satisfaction and its antecedents on consumer preference and intention. Adv Consum Res. 1981;8(1):88–93.
Bridges JF, Hauber AB, Marshall D, Lloyd A, Prosser LA, Regier DA, et al. Conjoint analysis applications in health—a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health. 2011;14(4):403–13.
Calvert M, Blazeby J, Altman DG, Revicki DA, Moher D, Brundage MD, et al. Reporting of patient-reported outcomes in randomized trials: the CONSORT PRO extension. JAMA. 2013;309(8):814–22.
Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Commun Health. 1998;52(6):377–84.
Lancsar E, Louviere J. Conducting discrete choice experiments to inform healthcare decision making: a user’s guide. Pharmacoeconomics. 2008;26(8):661–77.
Guimaraes C, Marra CA, Colley L, Gill S, Simpson S, Meneilly G, et al. Socioeconomic differences in preferences and willingness-to-pay for insulin delivery systems in type 1 and type 2 diabetes. Diabetes Technol Ther. 2009;11(9):567–73.
Guimaraes C, Marra CA, Colley L, Gill S, Simpson SH, Meneilly GS, et al. A valuation of patients’ willingness-to-pay for insulin delivery in diabetes. Int J Technol Assess Health Care. 2009;25(3):359–66.
Aristides M, Weston AR, FitzGerald P, Le Reun C, Maniadakis N. Patient preference and willingness-to-pay for Humalog Mix25 relative to humulin 30/70: a multicountry application of a discrete choice experiment. Value Health. 2004;7(4):442–54.
Hauber AB, Johnson FR, Sauriol L, Lescrauwaet B. Risking health to avoid injections: preferences of Canadians with type 2 diabetes. Diabetes Care. 2005;28(9):2243–5.
Hauber AB, Mohamed AF, Johnson FR, Falvey H. Treatment preferences and medication adherence of people with type 2 diabetes using oral glucose-lowering agents. Diabet Med. 2009;26(4):416–24.
Jendle J, Torffvit O, Ridderstrale M, Lammert M, Ericsson A, Bogelund M. Willingness to pay for health improvements associated with anti-diabetes treatments for people with type 2 diabetes. Curr Med Res Opin. 2010;26(4):917–23.
Polster M, Zanutto E, McDonald S, Conner C, Hammer M. A comparison of preferences for two GLP-1 products—liraglutide and exenatide—for the treatment of type 2 diabetes. J Med Econ. 2010;13(4):655–61.
Bogelund M, Vilsboll T, Faber J, Henriksen JE, Gjesing RP, Lammert M. Patient preferences for diabetes management among people with type 2 diabetes in Denmark—a discrete choice experiment. Curr Med Res Opin. 2011;27(11):2175–83.
Casciano R, Malangone E, Ramachandran A, Gagliardino JJ. A quantitative assessment of patient barriers to insulin. Int J Clin Pract. 2011;65(4):408–14.
Lloyd A, Nafees B, Barnett AH, Heller S, Ploug UJ, Lammert M, et al. Willingness to pay for improvements in chronic long-acting insulin therapy in individuals with type 1 or type 2 diabetes mellitus. Clin Ther. 2011;33(9):1258–67.
Brown SE, Meltzer DO, Chin MH, Huang ES. Perceptions of quality-of-life effects of treatments for diabetes mellitus in vulnerable and nonvulnerable older patients. J Am Geriatr Soc. 2008;56(7):1183–90.
Chancellor J, Aballea S, Lawrence A, Sheldon R, Cure S, Plun-Favreau J, et al. Preferences of patients with diabetes mellitus for inhaled versus injectable insulin regimens. Pharmacoeconomics. 2008;26(3):217–34.
Chin MH, Drum ML, Jin L, Shook ME, Huang ES, Meltzer DO. Variation in treatment preferences and care goals among older patients with diabetes and their physicians. Med Care. 2008;46(3):275–86.
Huang ES, Brown SE, Ewigman BG, Foley EC, Meltzer DO. Patient perceptions of quality of life with diabetes-related complications and treatments. Diabetes Care. 2007;30(10):2478–83.
MacKeigan LD, O’Brien BJ, Oh PI. Holistic versus composite preferences for lifetime treatment sequences for type 2 diabetes. Med Decis Making. 1999;19(2):113–21.
Boye KS, Matza LS, Walter KN, Van Brunt K, Palsgrove AC, Tynan A. Utilities and disutilities for attributes of injectable treatments for type 2 diabetes. Eur J Health Econ. 2011;12(3):219–30.
Matza LS, Boye KS, Yurgin N, Brewster-Jordan J, Mannix S, Shorr JM, et al. Utilities and disutilities for type 2 diabetes treatment-related attributes. Qual Life Res. 2007;16(7):1251–65.
Sadri H, MacKeigan LD, Leiter LA, Einarson TR. Willingness to pay for inhaled insulin: a contingent valuation approach. Pharmacoeconomics. 2005;23(12):1215–27.
Bergenstal RM, Freemantle N, Leyk M, Cutler GB Jr, Hayes RP, Muchmore DB. Does availability of AIR insulin increase insulin use and improve glycemic control in patients with type 2 diabetes? Diabetes Technol Ther. 2009;11(Suppl 2):S45–52.
Del Prato S, Blonde L, Martinez L, Goke B, Woo V, Millward A, et al. The effect of the availability of inhaled insulin on glycaemic control in patients with type 2 diabetes failing on oral therapy: the evaluation of Exubera as a therapeutic option on insulin initiation and improvement in glycaemic control in clinical practice (EXPERIENCE) trial. Diabet Med. 2008;25(6):662–70.
Martin JM, Llewelyn JA, Ristic S, Bates PC. Acceptability and safety of a new 3.0 ml re-usable insulin pen (HumaPen) in clinical use. Diabetes Nutr Metab. 1999;12(5):306–9.
Mullan RJ, Montori VM, Shah ND, Christianson TJ, Bryant SC, Guyatt GH, et al. The diabetes mellitus medication choice decision aid: a randomized trial. Arch Intern Med. 2009;169(17):1560–8.
Rosenstock J, Cappelleri JC, Bolinder B, Gerber RA. Patient satisfaction and glycemic control after 1 year with inhaled insulin (Exubera) in patients with type 1 or type 2 diabetes. Diabetes Care. 2004;27(6):1318–23.
Asakura T, Jensen KH. Comparison of intuitiveness, ease of use, and preference in two insulin pens. J Diabetes Sci Technol. 2009;3(2):312–9.
Asakura T, Seino H, Jensen KH. Patient acceptance and issues of education of two durable insulin pen devices. Diabetes Technol Ther. 2008;10(4):299–304.
Barnett AH, Bowen Jones D, Burden AC, Janes JM, Sinclair A, Small M, et al. Multicentre study to assess quality of life and glycaemic control of type 2 diabetic patients treated with insulin compared with oral hypoglycaemic agents. Pract Diabetes Int. 1996;13(6):179–83.
Bohannon NJ, Ohannesian JP, Burdan AL, Holcombe JH, Zagar A. Patient and physician satisfaction with the humulin/humalog pen, a new 3.0-mL prefilled pen device for insulin delivery. Clin Ther. 2000;22(9):1049–67.
Chan WB, Chow CC, Yeung VT, Chan JC, So WY, Cockram CS. Effect of insulin lispro on glycaemic control in Chinese diabetic patients receiving twice-daily regimens of insulin. Chin Med J (Engl). 2004;117(9):1404–7.
Clark PE, Valentine V, Bodie JN, Sarwat S. Ease of use and patient preference injection simulation study comparing two prefilled insulin pens. Curr Med Res Opin. 2010;26(7):1745–53.
D’Eliseo P, Blaauw J, Milicevic Z, Wyatt J, Ignaut DA, Malone JK. Patient acceptability of a new 3.0 ml pre-filled insulin pen. Curr Med Res Opin. 2000;16(2):125–33.
Diehl AK, Sugarek NJ, Bauer RL. Medication compliance in non-insulin-dependent diabetes: a randomized comparison of chlorpropamide and insulin. Diabetes Care. 1985;8(3):219–23.
Fox C, McKinnon C, Wall A, Lawton SA. Ability to handle, and patient preference for, insulin delivery devices in visually impaired patients with type 2 diabetes. Pract Diabetes Int. 2002;19(4):104–7.
Hansen B, Lilleore SK, Ter-Borch G. Needle with a novel attachment versus conventional screw-thread needles: a preference and usability test among adults with diabetes and impaired manual dexterity. Diabetes Technol Ther. 2011;13(5):579–85.
Hayes RP, Nakano M, Muchmore D, Schmitke J. Effect of standard (self-directed) training versus intensive training for Lilly/Alkermes human insulin inhalation powder delivery system on patient-reported outcomes and patient evaluation of the system. Diabetes Technol Ther. 2007;9(1):89–98.
Hirsch LJ, Gibney MA, Albanese J, Qu S, Kassler-Taub K, Klaff LJ, et al. Comparative glycemic control, safety and patient ratings for a new 4 mm × 32G insulin pen needle in adults with diabetes. Curr Med Res Opin. 2010;26(6):1531–41.
Israel-Bultman H, Hyllested-Winge J, Kolaczynski M, Steindorf J, Garon J. Comparison of preference for NovoPen® 4 with previous insulin pen treatments after 12 weeks in adult patients with type 1 and type 2 diabetes: a multicenter observational study. Clin Ther. 2011;33(3):346–57.
Korytkowski M, Bell D, Jacobsen C, Suwannasari R, FlexPen Study Team. A multicenter, randomized, open-label, comparative, two-period crossover trial of preference, efficacy, and safety profiles of a prefilled, disposable pen and conventional vial/syringe for insulin injection in patients with type 1 or 2 diabetes mellitus. Clin Ther. 2003;25(11):2836–48.
Larbig M, Forst T, Forst S, Lorra B, König K, Fittkau T, et al. Evaluation of the insulin application system autopen 24®. Pract Diabetes Int. 2005;22(9):364–6a.
Lee IT, Liau YJ, Lee WJ, Huang CN, Sheu WH. Continuous subcutaneous insulin infusion providing better glycemic control and quality of life in type 2 diabetic subjects hospitalized for marked hyperglycemia. J Eval Clin Pract. 2010;16(1):202–5.
Llewelyn J, Martin J, Bates P. Patient acceptability and safety of a new 3.0 ml prefilled insulin pen in a clinical setting. Pract Diabetes Int. 1999;16(3):79–86.
McKay M, Compion G, Lytzen L. A comparison of insulin injection needles on patients’ perceptions of pain, handling, and acceptability: a randomized, open-label, crossover study in subjects with diabetes. Diabetes Technol Ther. 2009;11(3):195–201.
Miwa T, Itoh R, Kobayashi T, Tanabe T, Shikuma J, Takahashi T, et al. Comparison of the effects of a new 32-gauge × 4-mm pen needle and a 32-gauge × 6-mm pen needle on glycemic control, safety, and patient ratings in Japanese adults with diabetes. Diabetes Technol Ther. 2012;14(12):1084–90.
Niskanen L, Jensen LE, Rastam J, Nygaard-Pedersen L, Erichsen K, Vora JP. Randomized, multinational, open-label, 2-period, crossover comparison of biphasic insulin aspart 30 and biphasic insulin lispro 25 and pen devices in adult patients with type 2 diabetes mellitus. Clin Ther. 2004;26(4):531–40.
Peyrot M, Rubin RR. Factors associated with persistence and resumption of insulin pen use for patients with type 2 diabetes. Diabetes Technol Ther. 2011;13(1):43–8.
Peyrot M, Rubin RR. Patient-reported outcomes in adults with type 2 diabetes using mealtime inhaled technosphere insulin and basal insulin versus premixed insulin. Diabetes Technol Ther. 2011;13(12):1201–6.
Peyrot M, Rubin RR. Validity and reliability of an instrument for assessing health-related quality of life and treatment preferences: the insulin delivery system rating questionnaire. Diabetes Care. 2005;28(1):53–8.
Schipper C, Musholt P, Niemeyer M, Qvist M, Loffler A, Forst T, et al. Patient device assessment evaluation of two insulin injection devices in a mixed cohort of insulin-treated patients with type 1 or type 2 diabetes mellitus. Curr Med Res Opin. 2012;28(8):1297–303.
Hayes RP, Bowman L, Monahan PO, Marrero DG, McHorney CA. Understanding diabetes medications from the perspective of patients with type 2 diabetes: prerequisite to medication concordance. Diabetes Educ. 2006;32(3):404–14.
Schwartz S, Hassman D, Shelmet J, Sievers R, Weinstein R, Liang J, et al. A multicenter, open-label, randomized, two-period crossover trial comparing glycemic control, satisfaction, and preference achieved with a 31 gauge × 6 mm needle versus a 29 gauge × 12.7 mm needle in obese patients with diabetes mellitus. Clin Ther. 2004;26(10):1663–78.
Polonsky WH, Fisher L, Hessler D, Bruhn D, Best JH. Patient perspectives on once-weekly medications for diabetes. Diabetes Obes Metab. 2011;13(2):144–9.
Puder JJ, Endrass J, Moriconi N, Keller U. How patients with insulin-treated type 1 and type 2 diabetes view their own and their physician’s treatment goals. Swiss Med Wkly. 2006;136(35–36):574–80.
Venekamp WJ, Kerr L, Dowsett SA, Johnson PA, Wimberley D, McKenzie C, et al. Functionality and acceptability of a new electronic insulin injection pen with a memory feature. Curr Med Res Opin. 2006;22(2):315–25.
Ristic S, Bates PC, Martin JM, Llewelyn JA. Acceptability of a reusable insulin pen, HumaPen ergo, by patients with type 1 and type 2 diabetes. Curr Med Res Opin. 2002;18(2):68–71.
Rubin RR, Peyrot M, Chen X, Frias JP. Patient-reported outcomes from a 16-week open-label, multicenter study of insulin pump therapy in patients with type 2 diabetes mellitus. Diabetes Technol Ther. 2010;12(11):901–6.
Stocks A, Perry S, Brydon P. HumaPen ergo®: a new 3.0 ml reusable insulin pen. Clin Drug Investig. 2001;21(5):319–24.
Summers KH, Szeinbach SL, Lenox SM. Preference for insulin delivery systems among current insulin users and nonusers. Clin Ther. 2004;26(9):1498–505.
Sucic M, Galic E, Cabrijan T, Ivandic A, Petrusic A, Wyatt J, et al. Patient acceptance and reliability of new humulin/humalog 3.0 ml prefilled insulin pen in ten Croatian diabetes centres. Med Sci Monit. 2002;8(3):PI21–6.
Szeinbach SL, Barnes JH, Summers KH, Lenox SM. Development of an instrument to assess expectations of and preference for an insulin injection pen compared with the vial and syringe. Clin Ther. 2004;26(4):590–7.
Stockl K, Ory C, Vanderplas A, Nicklasson L, Lyness W, Cobden D, et al. An evaluation of patient preference for an alternative insulin delivery system compared to standard vial and syringe. Curr Med Res Opin. 2007;23(1):133–46.
Rubin RR, Peyrot M. Psychometric properties of an instrument for assessing the experience of patients treated with inhaled insulin: the Inhaled Insulin Treatment Questionnaire (IITQ). Health Qual Life Outcomes. 2010;8:32.
Sommavilla B, Pietranera G. A randomized, open-label, comparative crossover handling trial between two durable pens in patients with type 1 or 2 diabetes mellitus. J Diabetes Sci Technol. 2011;5(5):1212–21.
Acknowledgments
The analysis upon which this publication is based was performed under contract number HHSF2232010000072C, entitled, “Partnership in Applied Comparative Effectiveness Science,” sponsored by the Food and Drug Administration, Department of Health and Human Services. The funder had no role in designing and conducting the study; collection, management, analysis, or interpretation of the data; or preparation/approval of the manuscript. SJ, NM, and JB conceptualized this paper; SJ, JB, EL, and TP developed the criteria for identifying preferences studies and rating their quality; SJ, EL, and TP conducted the systematic review; NM and TP provided clinical insights; and SJ and JB led the writing of the manuscript. SJ, EL, NM, TP, and JB all contributed to the writing of the manuscript and approved the final version. SJ, EL, NM, TP, and JB report no conflicts of interest. We thank Dr Jodi Segal for her advice and encouragement.
Author information
Authors and Affiliations
Corresponding author
Appendix 1: Example of Search Terms (PubMed)
Appendix 1: Example of Search Terms (PubMed)
“Diabetes mellitus, type 2”[mh] OR diabet*[tiab] OR “non-insulin dependent”[tiab] OR type-2[tiab] OR “type II”[tiab] OR “type 2”[tiab] “ketosis-resistant diabetes mellitus”[tw] OR “non-insulin-dependent diabetes mellitus”[tw] OR “type 2 diabetes mellitus”[tw] OR “stable diabetes mellitus”[tw] OR “maturity-onset diabetes mellitus”[tw] OR “maturity onset diabetes mellitus”[tw] OR “MODY”[tw] OR “NIDDM”[tw] OR “adult-onset diabetes mellitus”[tw]
AND
Treatment[tiab] OR management[tiab] OR pharmaceutical[tiab] OR drug therapy[mesh] OR medication[tiab]
AND
“Conjoint analysis” OR “satisfaction” OR “choice model” OR “stated preference” OR “discrete choice” OR DCE OR “decision analysis” OR preferences OR “multi-criteria decision analysis” OR MCDA OR “multi-attribute utility” OR “analytic hierarchy process” OR “trade off” OR “self-explicated” OR “best-worst scaling” OR utilities OR “preference weight” OR “willingness to pay” OR WTP OR “willingness to accept” OR “contingent valuation” OR priorities[tiab] OR valuation[tiab].
Rights and permissions
About this article
Cite this article
Joy, S.M., Little, E., Maruthur, N.M. et al. Patient Preferences for the Treatment of Type 2 Diabetes: A Scoping Review. PharmacoEconomics 31, 877–892 (2013). https://doi.org/10.1007/s40273-013-0089-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-013-0089-7