Abstract
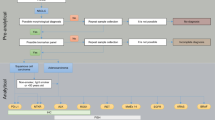
Patients with advanced non-small-cell lung cancer (NSCLC) carrying epidermal growth factor receptor (EGFR) mutations can now have specific treatment based on the result of biomarker analysis and patients with rearrangements of the anaplastic lymphoma kinase (ALK) gene will probably soon be able to. This will give them better quality of life and progression-free survival than conventional chemotherapy. This consensus statement was conceived as a joint initiative of the Spanish Society of Medical Oncology (SEOM) and the Spanish Society of Pathology (SEAP), and makes diagnostic and treatment recommendations for advanced NSCLC patients based on the scientific evidence on biomarker use. It therefore provides an opportunity to improve healthcare efficiency and resource use, which will undoubtedly benefit these patients. Although this field is in continuous evolution, at present, with the available data, this panel of experts recommends that all patients with advanced NSCLC of non-squamous cell subtype, or non-smokers regardless of the histological subtype, should be tested for EGFR gene mutations within a maximum of 7 days from the pathological diagnosis. Involved laboratories must participate in external quality control programmes. In contrast, ALK gene rearrangements should only be tested in the context of a clinical trial, although the promising data obtained will certainly justify in the near future its routine testing in patients with no EGFR mutations. Lastly, routine testing for other molecular abnormalities is not considered necessary in the current clinical practice.
Similar content being viewed by others
References
Jemal A, Bray F, Center MM et al (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Pao W, Girard N (2011) New driver mutations in non-small-cell lung cancer. Lancet Oncol 12:175–180
Lynch TJ, Bell DW, Sordella R et al (2004) Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-smallcell lung cancer to gefitinib. N Engl J Med 350: 2129–2139
Paez JG, Janne PA, Lee JC et al (2004) EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304:1497–1500
Rosell R, Moran T, Queralt C et al (2009) Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 361:958–967
Mok TS, Wu YL, Thongprasert S et al (2009) Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361:947–957
Lee JS, Park K, Kim SW et al (2009) A randomized phase III study of gefitinib (IRESSA?) versus standard chemotherapy (gemcitabine plus cisplatin) as a first-line treatment for never-smokers with advanced or metastatic adenocarcinoma of the lung. J Thorac Oncol 4[Suppl 1]:Abstract PRS 4
Mitsudomi T, Morita S, Yatabe Y et al (2010) Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 11:121–128
Maemondo M, Inoue A, Kobayashi K et al (2010) Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362: 2380–2388
Zhou C, Wu YL, Chen G et al (2010) Efficacy results from the randomized phase III OPTIMAL (CTONG 0802) study comparing first-line erlotinib versus carboplatin (CBDCA) plus gemcitabine (GEM), in Chinese advanced non-small-cell lung cancer (NSCLC) patients (PTS) with EGFR activating mutations. Ann Oncol 21:Abstract LBA13
Rosell R, Gervais R, Vergnenegre A et al (2011) Erlotinib versus chemotherapy (CT) in advanced non-small cell lung cancer (NSCLC) patients (p) with epidermal growth factor receptor (EGFR) mutations: Interim results of the European Erlotinib Versus Chemotherapy (EURTAC) phase III randomized trial. In: ASCO Annual Meeting, Abstract 7503. Chicago, IL, EEUU
Felip E, Gridelli C, Baas P et al (2011) Metastatic non-small-cell lung cancer: consensus on pathology and molecular tests, first-line, second-line, and third-line therapy: 1st ESMO Consensus Conference in Lung Cancer; Lugano 2010. Ann Oncol 22:1507–1519
Keedy VL, Temin S, Somerfield MR et al (2011) American Society of Clinical Oncology provisional clinical opinion: epidermal growth factor receptor (EGFR) Mutation testing for patients with advanced non-small-cell lung cancer considering first-line EGFR tyrosine kinase inhibitor therapy. J Clin Oncol 29:2121–2127
Trigo Perez JM, Garrido Lopez P, Felip Font E et al (2010) SEOM clinical guidelines for the treatment of non-small-cell lung cancer: an updated edition. Clin Transl Oncol 12:735–741
Morris SW, Kirstein MN, Valentine MB et al (1994) Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science 263:1281–1284
Chiarle R, Voena C, Ambrogio C et al (2008) The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat Rev Cancer 8:11–23
Rikova K, Guo A, Zeng Q et al (2007) Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell 131:1190–1203
Soda M, Choi YL, Enomoto M et al (2007) Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 448: 561–566
Weinstein IB, Joe A (2008) Oncogene addiction. Cancer Res 68:3077–3080; discussion 3080
Sasaki T, Rodig SJ, Chirieac LR et al (2010) The biology and treatment of EML4-ALK non-small cell lung cancer. Eur J Cancer 46:1773–1780
Shaw AT, Yeap BY, Mino-Kenudson M et al (2009) Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 27:4247–4253
Kwak EL, Bang YJ, Camidge DR et al (2010) Anaplastic lymphoma kinase inhibition in nonsmall-cell lung cancer. N Engl J Med 363:1693–1703
Ding L, Getz G, Wheeler DA et al (2008) Somatic mutations affect key pathways in lung adenocarcinoma. Nature 455:1069–1075
Beau-Faller M, Ruppert AM, Voegeli AC et al (2008) MET gene copy number in non-small cell lung cancer: molecular analysis in a targeted tyrosine kinase inhibitor naive cohort. J Thorac Oncol 3:331–339
Eder JP, Vande Woude GF, Boerner SA et al (2009) Novel therapeutic inhibitors of the c-Met signaling pathway in cancer. Clin Cancer Res 15: 2207–2214
Hirsch FR, Langer CJ (2004) The role of HER2/neu expression and trastuzumab in non-small cell lung cancer. Semin Oncol 31:75–82
Perera SA, Li D, Shimamura T et al (2009) HER2YVMA drives rapid development of adenosquamous lung tumors in mice that are sensitive to BIBW2992 and rapamycin combination therapy. Proc Natl Acad Sci U S A 106:474–479
Shimamura T, Ji H, Minami Y et al (2006) Nonsmall-cell lung cancer and Ba/F3 transformed cells harboring the ERBB2 G776insV_G/C mutation are sensitive to the dual-specific epidermal growth factor receptor and ERBB2 inhibitor HKI-272. Cancer Res 66:6487–6491
Wang SE, Narasanna A, Perez-Torres M et al (2006) HER2 kinase domain mutation results in constitutive phosphorylation and activation of HER2 and EGFR and resistance to EGFR tyrosine kinase inhibitors. Cancer Cell 10:25–38
Paik PK, Arcila ME, Fara M et al (2011) Clinical characteristics of patients with lung adenocarcinomas harboring BRAF mutations. J Clin Oncol 29: 2046–2051
Okudela K, Suzuki M, Kageyama S et al (2007) PIK3CA mutation and amplification in human lung cancer. Pathol Int 57:664–671
Kawano O, Sasaki H, Endo K et al (2006) PIK3-CA mutation status in Japanese lung cancer patients. Lung Cancer 54:209–215
Angulo B, Suarez-Gauthier A, Lopez-Rios F et al (2008) Expression signatures in lung cancer reveal a profile for EGFR-mutant tumours and identify selective PIK3CA overexpression by gene amplification. J Pathol 214:347–356
Travis WD, Brambilla E, Müller-Hermelink HK, Harris CC (2004) Pathology and genetics of tumours of the lung, pleura, thymus and heart. WHO classification of tumours. IARC Press, Lyon
Salido M, Pijuan L, Martinez-Aviles L et al (2011) Increased ALK gene copy number and amplification are frequent in non-small cell lung cancer. J Thorac Oncol 6:21–27
Sharma SV, Haber DA, Settleman J (2010) Cell line-based platforms to evaluate the therapeutic efficacy of candidate anticancer agents. Nat Rev Cancer 10:241–253
SEAP-IAP (2011) Garantía de Calidad en Patología de la Sociedad Española de Anatomía Patológica y la División Española del International Academy of Pathology. http://www.seap.es/gcp/molecular/index.asp
Travis WD, Brambilla E, Noguchi M et al (2011) International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 6:244–285
Mukhopadhyay S, Katzenstein AL (2011) Subclassification of non-small cell lung carcinomas lacking morphologic differentiation on biopsy specimens: utility of an immunohistochemical panel containing TTF-1, napsin A, p63, and CK5/6. Am J Surg Pathol 35:15–25
Boldrini L, Gisfredi S, Ursino S et al (2007) Mutational analysis in cytological specimens of advanced lung adenocarcinoma: a sensitive method for molecular diagnosis. J Thorac Oncol 2: 1086–1090
Ladanyi M, Pao W (2008) Lung adenocarcinoma: guiding EGFR-targeted therapy and beyond. Mod Pathol 21[Suppl 2]:S16–22
Pirker R, Herth FJ, Kerr KM et al (2010) Consensus for EGFR mutation testing in non-small cell lung cancer: results from a European workshop. J Thorac Oncol 5:1706–1713
Angulo B, Garcia-Garcia E, Martinez R et al (2010) A commercial real-time PCR kit provides greater sensitivity than direct sequencing to detect KRAS mutations: a morphology-based approach in colorectal carcinoma. J Mol Diagn 12: 292–299
Brevet M, Arcila M, Ladanyi M (2010) Assessment of EGFR mutation status in lung adenocarcinoma by immunohistochemistry using antibodies specific to the two major forms of mutant EGFR. J Mol Diagn 12:169–176
Conde E, Angulo B, Tang M et al (2006) Molecular context of the EGFR mutations: evidence for the activation of mTOR/S6K signaling. Clin Cancer Res 12:710–717
Kato Y, Peled N, Wynes MW et al (2010) Novel epidermal growth factor receptor mutation-specific antibodies for non-small cell lung cancer: immunohistochemistry as a possible screening method for epidermal growth factor receptor mutations. J Thorac Oncol 5:1551–1558
Kawahara A, Yamamoto C, Nakashima K et al (2010) Molecular diagnosis of activating EGFR mutations in non-small cell lung cancer using mutation-specific antibodies for immunohistochemical analysis. Clin Cancer Res 16:3163–3170
Marchetti A, Normanno N, Pinto C et al (2010) Recommendations for mutational analysis of EGFR in lung carcinoma. Pathologica 102:119–126
Pan Q, Pao W, Ladanyi M (2005) Rapid polymerase chain reaction-based detection of epidermal growth factor receptor gene mutations in lung adenocarcinomas. J Mol Diagn 7:396–403
Pao W, Ladanyi M (2007) Epidermal growth factor receptor mutation testing in lung cancer: searching for the ideal method. Clin Cancer Res 13:4954–4955
Yu J, Kane S, Wu J et al (2009) Mutation-specific antibodies for the detection of EGFR mutations in non-small-cell lung cancer. Clin Cancer Res 15: 3023–3028
Travis WD, Rekhtman N (2011) Pathological diagnosis and classification of lung cancer in small biopsies and cytology: strategic management of tissue for molecular testing. Semin Respir Crit Care Med 32:22–31
Billah S, Stewart J, Staerkel G et al (2011) EGFR and KRAS mutations in lung carcinoma: molecular testing by using cytology specimens. Cancer Cytopathol 119:111–117
Author information
Authors and Affiliations
Corresponding author
Additional information
The affiliations are listed at the end of the article
Rights and permissions
About this article
Cite this article
Garrido, P., de Castro, J., Concha, Á. et al. Guidelines for biomarker testing in advanced non-small-cell lung cancer. A national consensus of the Spanish Society of Medical Oncology (SEOM) and the Spanish Society of Pathology (SEAP). Clin Transl Oncol 14, 338–349 (2012). https://doi.org/10.1007/s12094-012-0806-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-012-0806-2