Abstract
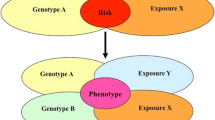
Since sarcoidosis was first described more than a century ago, the etiologic determinants causing this disease remain uncertain. Studies suggest that genetic, host immunologic, and environmental factors interact together to cause sarcoidosis. Immunologic characteristics of sarcoidosis include non-caseating granulomas, enhanced local expression of T helper-1 (and often Th17) cytokines and chemokines, dysfunctional regulatory T-cell responses, dysregulated Toll-like receptor signaling, and oligoclonal expansion of CD4+ T cells consistent with chronic antigenic stimulation. Multiple environmental agents have been suggested to cause sarcoidosis. Studies from several groups implicate mycobacterial or propionibacterial organisms in the etiology of sarcoidosis based on tissue analyses and immunologic responses in sarcoidosis patients. Despite these studies, there is no consensus on the nature of a microbial pathogenesis of sarcoidosis. Some groups postulate sarcoidosis is caused by an active viable replicating infection while other groups contend there is no clinical, pathologic, or microbiologic evidence for such a pathogenic mechanism. The authors posit a novel hypothesis that proposes that sarcoidosis is triggered by a hyperimmune Th1 response to pathogenic microbial and tissue antigens associated with the aberrant aggregation of serum amyloid A within granulomas, which promotes progressive chronic granulomatous inflammation in the absence of ongoing infection.

Similar content being viewed by others
References
(1999) Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med 160(2): 736–755
Clay H, Davis JM, Beery D, Huttenlocher A, Lyons SE, Ramakrishnan L (2007) Dichotomous role of the macrophage in early Mycobacterium marinum infection of the zebrafish. Cell Host Microbe 2(1):29–39
Moller DR, Forman JD, Liu MC, Noble PW, Greenlee BM, Vyas P et al (1996) Enhanced expression of IL-12 associated with Th1 cytokine profiles in active pulmonary sarcoidosis. J Immunol 156(12):4952–4960
Greene CM, Meachery G, Taggart CC, Rooney CP, Coakley R, O'Neill SJ et al (2000) Role of IL-18 in CD4+ T lymphocyte activation in sarcoidosis. J Immunol 165(8):4718–4724
Shigehara K, Shijubo N, Ohmichi M, Takahashi R, Kon S, Okamura H et al (2001) IL-12 and IL-18 are increased and stimulate IFN-gamma production in sarcoid lungs. J Immunol 166(1):642–649
Facco M, Cabrelle A, Teramo A, Olivieri V, Gnoato M, Teolato S et al (2011) Sarcoidosis is a Th1/Th17 multisystem disorder. Thorax 66(2):144–150
Ten Berge B, Paats MS, Bergen IM, van den Blink B, Hoogsteden HC, Lambrecht BN et al (2012) Increased IL-17A expression in granulomas and in circulating memory T cells in sarcoidosis. Rheumatology (Oxford) 51(1):37–46
Richmond BW, Ploetze K, Isom J, Chambers-Harris I, Braun NA, Taylor T et al (2013) Sarcoidosis Th17 cells are ESAT-6 antigen specific but demonstrate reduced IFN-gamma expression. J Clin Immunol 33(2):446–455
Romagnani S (2006) Regulation of the T cell response. Clin Exp Allergy 36(11):1357–1366
Simonian PL, Roark CL, Wehrmann F, Lanham AK, Diaz de Valle F, Born WK et al (2009) Th17-polarized immune response in a murine model of hypersensitivity pneumonitis and lung fibrosis. J Immunol 182(1):657–665
Joshi AD, Fong DJ, Oak SR, Trujillo G, Flaherty KR, Martinez FJ et al (2009) Interleukin-17-mediated immunopathogenesis in experimental hypersensitivity pneumonitis. Am J Respir Crit Care Med 179(8):705–716
Su R, Li MM, Bhakta NR, Solberg OD, Darnell EP, Ramstein J et al (2014) Longitudinal analysis of sarcoidosis blood transcriptomic signatures and disease outcomes. Eur Respir J
Miyara M, Amoura Z, Parizot C, Badoual C, Dorgham K, Trad S et al (2006) The immune paradox of sarcoidosis and regulatory T cells. J Exp Med 203(2):359–370
Taflin C, Miyara M, Nochy D, Valeyre D, Naccache JM, Altare F et al (2009) FoxP3+ regulatory T cells suppress early stages of granuloma formation but have little impact on sarcoidosis lesions. Am J Pathol 174(2):497–508
Rappl G, Pabst S, Riemann D, Schmidt A, Wickenhauser C, Schutte W et al (2011) Regulatory T cells with reduced repressor capacities are extensively amplified in pulmonary sarcoid lesions and sustain granuloma formation. Clin Immunol 140(1):71–83
Prasse A, Zissel G, Lutzen N, Schupp J, Schmiedlin R, Gonzalez-Rey E et al (2010) Inhaled vasoactive intestinal peptide exerts immunoregulatory effects in sarcoidosis. Am J Respir Crit Care Med 182(4):540–548
Lee NS, Barber L, Kanchwala A, Childs CJ, Kataria YP, Judson MA et al (2011) Low levels of NF-kappaB/p65 mark anergic CD4+ T cells and correlate with disease severity in sarcoidosis. Clin Vaccine Immunol 18(2):223–234
Braun NA, Celada LJ, Herazo-Maya JD, Abraham S, Shaginurova G, Sevin CM et al (2014) Blockade of the programmed death-1 pathway restores sarcoidosis CD4(+) T-cell proliferative capacity. Am J Respir Crit Care Med 190(5):560–571
Sverrild A, Backer V, Kyvik KO, Kaprio J, Milman N, Svendsen CB et al (2008) Heredity in sarcoidosis: a registry-based twin study. Thorax 63(10):894–896
Rybicki BA, Iannuzzi MC, Frederick MM, Thompson BW, Rossman MD, Bresnitz EA et al (2001) Familial aggregation of sarcoidosis. A case–control etiologic study of sarcoidosis (ACCESS). Am J Respir Crit Care Med 164(11):2085–2091
Sato H, Woodhead FA, Ahmad T, Grutters JC, Spagnolo P, van den Bosch JM et al (2010) Sarcoidosis HLA class II genotyping distinguishes differences of clinical phenotype across ethnic groups. Hum Mol Genet 19(20):4100–4111
Darlington P, Gabrielsen A, Sorensson P, Tallstedt L, Padyukov L, Eklund A et al (2014) HLA-alleles associated with increased risk for extra-pulmonary involvement in sarcoidosis. Tissue Antigens 83(4):267–272
Ozyilmaz E, Goruroglu Ozturk O, Yunsel D, Deniz A, Hanta I, Kuleci S et al (2014) Could HLA-DR B1*11 allele be a clue for predicting extra-pulmonary sarcoidosis? Sarcoidosis Vasc Diffuse Lung Dis 31(2):154–162
Bogunia-Kubik K, Tomeczko J, Suchnicki K, Lange A (2001) HLA-DRB1*03, DRB1*11 or DRB1*12 and their respective DRB3 specificities in clinical variants of sarcoidosis. Tissue Antigens 57(1):87–90
Grubic Z, Peros-Golubicic T, Stingl K, Zunec R (2011) The investigation of HLA microsatellites influence in predisposition to sarcoidosis among Croatians. Sarcoidosis Vasc Diffuse Lung Dis 28(1):18–26
Ishihara M, Ohno S, Ishida T, Ando H, Naruse T, Nose Y et al (1994) Molecular genetic studies of HLA class II alleles in sarcoidosis. Tissue Antigens 43(4):238–241
Sharma SK, Balamurugan A, Pandey RM, Saha PK, Mehra NK (2003) Human leukocyte antigen-DR alleles influence the clinical course of pulmonary sarcoidosis in Asian Indians. Am J Respir Cell Mol Biol 29(2):225–231
Zhou Y, Shen L, Zhang Y, Jiang D, Li H (2011) Human leukocyte antigen-A, -B, and -DRB1 alleles and sarcoidosis in Chinese Han subjects. Hum Immunol 72(7):571–575
Grosser M, Luther T, Fuessel M, Bickhardt J, Magdolen V, Baretton G (2005) Clinical course of sarcoidosis in dependence on HLA-DRB1 allele frequencies, inflammatory markers, and the presence of M. tuberculosis DNA fragments. Sarcoidosis Vasc Diffuse Lung Dis 22(1):66–74
Dubaniewicz A, Dubaniewicz-Wybieralska M, Moszkowska G, Sternau A, Dubaniewicz A (2006) Comparative analysis of DR and DQ alleles occurrence in sarcoidosis and tuberculosis in the same ethnic group: preliminary study. Sarcoidosis Vasc Diffuse Lung Dis 23(3):180–189
Rossman MD, Thompson B, Frederick M, Iannuzzi MC, Rybicki BA, Pander JP et al (2008) HLA and environmental interactions in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 25(2):125–132
Grunewald J, Hultman T, Bucht A, Eklund A, Wigzell H (1995) Restricted usage of T cell receptor V alpha/J alpha gene segments with different nucleotide but identical amino acid sequences in HLA-DR3+ sarcoidosis patients. Mol Med 1(3):287–296
Darlington P, Haugom-Olsen H, von Sivers K, Wahlstrom J, Runold M, Svjatoha V et al (2012) T-cell phenotypes in bronchoalveolar lavage fluid, blood and lymph nodes in pulmonary sarcoidosis—indication for an airborne antigen as the triggering factor in sarcoidosis. J Intern Med 272(5):465–471
Kuroda H, Saijo Y, Fujiuchi S, Takeda H, Ohsaki Y, Hasebe N (2013) Relationship between cytokine single nucleotide polymorphisms and sarcoidosis among Japanese subjects. Sarcoidosis Vasc Diffuse Lung Dis 30(1):36–42
Seitzer U, Swider C, Stuber F, Suchnicki K, Lange A, Richter E et al (1997) Tumour necrosis factor alpha promoter gene polymorphism in sarcoidosis. Cytokine 9(10):787–790
Fischer A, Nothnagel M, Schurmann M, Muller-Quernheim J, Schreiber S, Hofmann S (2010) A genome-wide linkage analysis in 181 German sarcoidosis families using clustered biallelic markers. Chest 138(1):151–157
Chen ES, Song Z, Willett MH, Heine S, Yung RC, Liu MC et al (2010) Serum amyloid A regulates granulomatous inflammation in sarcoidosis through Toll-like receptor-2. Am J Respir Crit Care Med 181(4):360–373
Wiken M, Grunewald J, Eklund A, Wahlstrom J (2009) Higher monocyte expression of TLR2 and TLR4, and enhanced pro-inflammatory synergy of TLR2 with NOD2 stimulation in sarcoidosis. J Clin Immunol 29(1):78–89
Gabrilovich MI, Walrath J, van Lunteren J, Nethery D, Seifu M, Kern JA et al (2013) Disordered Toll-like receptor 2 responses in the pathogenesis of pulmonary sarcoidosis. Clin Exp Immunol 173(3):512–522
Veltkamp M, van Moorsel CH, Rijkers GT, Ruven HJ, Grutters JC (2012) Genetic variation in the Toll-like receptor gene cluster (TLR10-TLR1-TLR6) influences disease course in sarcoidosis. Tissue Antigens 79(1):25–32
Veltkamp M, Wijnen PA, van Moorsel CH, Rijkers GT, Ruven HJ, Heron M et al (2007) Linkage between Toll-like receptor (TLR) 2 promotor and intron polymorphisms: functional effects and relevance to sarcoidosis. Clin Exp Immunol 149(3):453–462
Sato M, Kawagoe T, Meguro A, Ota M, Katsuyama Y, Ishihara M et al (2011) Toll-like receptor 2 (TLR2) gene polymorphisms are not associated with sarcoidosis in the Japanese population. Mol Vis 17:731–736
Rastogi R, Du W, Ju D, Pirockinaite G, Liu Y, Nunez G et al (2011) Dysregulation of p38 and MKP-1 in response to NOD1/TLR4 stimulation in sarcoid bronchoalveolar cells. Am J Respir Crit Care Med 183(4):500–510
Kanazawa N, Okafuji I, Kambe N, Nishikomori R, Nakata-Hizume M, Nagai S et al (2005) Early-onset sarcoidosis and CARD15 mutations with constitutive nuclear factor-kappaB activation: common genetic etiology with Blau syndrome. Blood 105(3):1195–1197
Martin TM, Doyle TM, Smith JR, Dinulescu D, Rust K, Rosenbaum JT (2003) Uveitis in patients with sarcoidosis is not associated with mutations in NOD2 (CARD15). Am J Ophthalmol 136(5):933–935
Schurmann M, Valentonyte R, Hampe J, Muller-Quernheim J, Schwinger E, Schreiber S (2003) CARD15 gene mutations in sarcoidosis. Eur Respir J: Off J Eur Soc Clin Respir Physiol 22(5):748–754
Milman N, Nielsen OH, Hviid TV, Fenger K (2007) CARD15 single nucleotide polymorphisms 8, 12 and 13 are not increased in ethnic Danes with sarcoidosis. Respiration 74(1):76–79
Akahoshi M, Ishihara M, Namba K, Kitaichi N, Ando Y, Takenaka S et al (2008) Mutation screening of the CARD15 gene in sarcoidosis. Tissue Antigens 71(6):564–567
Campo I, Morbini P, Zorzetto M, Tinelli C, Brunetta E, Villa C et al (2007) Expression of receptor for advanced glycation end products in sarcoid granulomas. Am J Respir Crit Care Med 175(5):498–506
Kim MH, Choi YW, Choi HY, Myung KB, Cho SN (2006) The expression of RAGE and EN-RAGE in leprosy. Br J Dermatol 154(4):594–601
Sakaguchi M, Murata H, Yamamoto K, Ono T, Sakaguchi Y, Motoyama A et al (2011) TIRAP, an adaptor protein for TLR2/4, transduces a signal from RAGE phosphorylated upon ligand binding. PLoS One 6(8):e23132
Akira S (2003) Mammalian Toll-like receptors. Curr Opin Immunol 15(1):5–11
Daniil Z, Mollaki V, Malli F, Koutsokera A, Antoniou KM, Rodopoulou P et al (2013) Polymorphisms and haplotypes in MyD88 are associated with the development of sarcoidosis: a candidate-gene association study. Mol Biol Rep 40(7):4281–4286
Belkaid Y (2007) Regulatory T, cells and infection: a dangerous necessity. Nat Rev Immunol 7(11):875–888
Trinath J, Maddur MS, Kaveri SV, Balaji KN, Bayry J (2012) Mycobacterium tuberculosis promotes regulatory T-cell expansion via induction of programmed death-1 ligand 1 (PD-L1, CD274) on dendritic cells. J Infect Dis 205(4):694–696
Wilsher ML (1998) Seasonal clustering of sarcoidosis presenting with erythema nodosum. Eur Respir J 12(5):1197–1199
Izbicki G, Chavko R, Banauch GI, Weiden MD, Berger KI, Aldrich TK et al (2007) World Trade Center “sarcoid-like” granulomatous pulmonary disease in New York City Fire Department rescue workers. Chest 131(5):1414–1423
Kreider ME, Christie JD, Thompson B, Newman L, Rose C, Barnard J et al (2005) Relationship of environmental exposures to the clinical phenotype of sarcoidosis. Chest 128(1):207–215
Newman LS, Rose CS, Bresnitz EA, Rossman MD, Barnard J, Frederick M et al (2004) A case control etiologic study of sarcoidosis: environmental and occupational risk factors. Am J Respir Crit Care Med 170(12):1324–1330
Barnard J, Rose C, Newman L, Canner M, Martyny J, McCammon C et al (2005) Job and industry classifications associated with sarcoidosis in A Case–Control Etiologic Study of Sarcoidosis (ACCESS). J Occup Environ Med 47(3):226–234
Newman LS (1996) Immunology, genetics, and epidemiology of beryllium disease. Chest 109(3 Suppl):40S–43S
Muller-Quernheim J, Gaede KI, Fireman E, Zissel G (2006) Diagnoses of chronic beryllium disease within cohorts of sarcoidosis patients. Eur Respir J 27(6):1190–1195
Mack DG, Falta MT, McKee AS, Martin AK, Simonian PL, Crawford F et al (2014) Regulatory T cells modulate granulomatous inflammation in an HLA-DP2 transgenic murine model of beryllium-induced disease. Proc Natl Acad Sci U S A 111(23):8553–8558
Respiratory illness in workers exposed to metalworking fluid contaminated with nontuberculous mycobacteria—Ohio, 2001. MMWR Morb Mortal Wkly Rep (2002) 51(16): 349–352
Shvedova AA, Kisin ER, Mercer R, Murray AR, Johnson VJ, Potapovich AI et al (2005) Unusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. Am J Physiol Lung Cell Mol Physiol 289(5):L698–L708
Huizar I, Malur A, Patel J, McPeek M, Dobbs L, Wingard C et al (2013) The role of PPARgamma in carbon nanotube-elicited granulomatous lung inflammation. Respir Res 14:7
Barna BP, Huizar I, Malur A, McPeek M, Marshall I, Jacob M et al (2013) Carbon nanotube-induced pulmonary granulomatous disease: Twist1 and alveolar macrophage M1 activation. Int J Mol Sci 14(12):23858–23871
Judson MA, Baughman RP (2014) How many organs need to be involved to diagnose sarcoidosis?: An unanswered question that, hopefully, will become irrelevant. Sarcoidosis Vasc Diffuse Lung Dis 31(1):6–7
Heyll A, Meckenstock G, Aul C, Sohngen D, Borchard F, Hadding U et al (1994) Possible transmission of sarcoidosis via allogeneic bone marrow transplantation. Bone Marrow Transplant 14(1):161–164
Sundar KM, Carveth HJ, Gosselin MV, Beatty PG, Colby TV, Hoidal JR (2001) Granulomatous pneumonitis following bone marrow transplantation. Bone Marrow Transplant 28(6):627–630
Gooneratne L, Lim ZY, Vivier A, Salisbury JR, Knisely AS, Ho AY et al (2007) Sarcoidosis as an unusual cause of hepatic dysfunction following reduced intensity conditioned allogeneic stem cell transplantation. Bone Marrow Transplant 39(8):511–512
Morita R, Hashino S, Kubota K, Onozawa M, Kahata K, Kondo T et al (2009) Donor cell-derived sarcoidosis after allogeneic BMT. Bone Marrow Transplant 43(6):507–508
Johnson BA, Duncan SR, Ohori NP, Paradis IL, Yousem SA, Grgurich WF et al (1993) Recurrence of sarcoidosis in pulmonary allograft recipients. Am Rev Respir Dis 148(5):1373–1377
Ramakers K, De Wever W, Coolen J, Verschakelen J (2012) Recurrent sarcoidosis after lung transplantation. JBR-BTR 95(6):368
Fidler HM, Hadziyannis SJ, Dhillon AP, Sherlock S, Burroughs AK (1997) Recurrent hepatic sarcoidosis following liver transplantation. Transplant Proc 29(5):2509–2510
Vanatta JM, Modanlou KA, Dean AG, Nezakatgoo N, Campos L, Nair S et al (2011) Outcomes of orthotopic liver transplantation for hepatic sarcoidosis: an analysis of the United Network for Organ Sharing/Organ Procurement and Transplantation Network data files for a comparative study with cholestatic liver diseases. Liver Transpl 17(9):1027–1034
Oni AA, Hershberger RE, Norman DJ, Ray J, Hovaguimian H, Cobanoglu AM et al (1992) Recurrence of sarcoidosis in a cardiac allograft: control with augmented corticosteroids. J Heart Lung Transplant 11(2 Pt 1):367–369
Akashi H, Kato TS, Takayama H, Naka Y, Farr M, Mancini D et al (2012) Outcome of patients with cardiac sarcoidosis undergoing cardiac transplantation—single-center retrospective analysis. J Cardiol 60(5):407–410
Shen SY, Hall-Craggs M, Posner JN, Shabazz B (1986) Recurrent sarcoid granulomatous nephritis and reactive tuberculin skin test in a renal transplant recipient. Am J Med 80(4):699–702
Aouizerate J, Matignon M, Kamar N, Thervet E, Randoux C, Moulin B et al (2010) Renal transplantation in patients with sarcoidosis: a French multicenter study. Clin J Am Soc Nephrol 5(11):2101–2108
Milman N, Andersen CB, Burton CM, Iversen M (2005) Recurrent sarcoid granulomas in a transplanted lung derive from recipient immune cells. Eur Respir J 26(3):549–552
Pukiat S, McCarthy PL Jr, Hahn T, Morrison C, Shanahan T, Qiu J et al (2011) Sarcoidosis-associated MHC Ags and the development of cutaneous and nodal granulomas following allogeneic hematopoietic cell transplant. Bone Marrow Transplant 46(7):1032–1034
Munro CS, Mitchell DN (1987) The Kveim response: still useful, still a puzzle. Thorax 42(5):321–331
Kooij R, Gerritsen T (1958) On the nature of the Mitsuda and the Kveim reaction. Dermatologica 116(1):1–27
Klein JT, Horn TD, Forman JD, Silver RF, Teirstein AS, Moller DR (1995) Selection of oligoclonal V beta-specific T cells in the intradermal response to Kveim-Siltzbach reagent in individuals with sarcoidosis. J Immunol 154(3):1450–1460
Segal JL, Thompson JF, Charter RA (2012) A novel immunogen to modulate cytokine production and promote immune system reconstitution in HIV-AIDS. Am J Ther 19(5):317–323
Milman N, Lisby G, Friis S, Kemp L (2004) Prolonged culture for mycobacteria in mediastinal lymph nodes from patients with pulmonary sarcoidosis. A negative study. Sarcoidosis Vasc Diffuse Lung Dis 21(1):25–28
Brown ST, Brett I, Almenoff PL, Lesser M, Terrin M, Teirstein AS (2003) Recovery of cell wall-deficient organisms from blood does not distinguish between patients with sarcoidosis and control subjects. Chest 123(2):413–417
Gupta D, Agarwal R, Aggarwal AN, Jindal SK (2007) Molecular evidence for the role of mycobacteria in sarcoidosis: a meta-analysis. Eur Respir J 30(3):508–516
Zhou Y, Li HP, Li QH, Zheng H, Zhang RX, Chen G et al (2008) Differentiation of sarcoidosis from tuberculosis using real-time PCR assay for the detection and quantification of Mycobacterium tuberculosis. Sarcoidosis Vasculitis Diffuse Lung Dis: Off J WASOG / World Assoc Sarcoidosis Other Granulomatous Disord 25(2):93–99
Song Z, Marzilli L, Greenlee BM, Chen ES, Silver RF, Askin FB et al (2005) Mycobacterial catalase-peroxidase is a tissue antigen and target of the adaptive immune response in systemic sarcoidosis. J Exp Med 201(5):755–767
Chen ES, Wahlstrom J, Song Z, Willett MH, Wiken M, Yung RC et al (2008) T cell responses to mycobacterial catalase-peroxidase profile a pathogenic antigen in systemic sarcoidosis. J Immunol 181(12):8784–8796
Hanngren A, Odham G, Eklund A, Hoffner S, Stjernberg N, Westerdahl G (1987) Tuberculostearic acid in lymph nodes from patients with sarcoidosis. Sarcoidosis 4(2):101–104
Dubaniewicz A, Dubaniewicz-Wybieralska M, Sternau A, Zwolska Z, Izycka-Swieszewska E, Augustynowicz-Kopec E et al (2006) Mycobacterium tuberculosis complex and mycobacterial heat shock proteins in lymph node tissue from patients with pulmonary sarcoidosis. J Clin Microbiol 44(9):3448–3451
Oswald-Richter KA, Beachboard DC, Seeley EH, Abraham S, Shepherd BE, Jenkins CA et al (2012) Dual analysis for mycobacteria and propionibacteria in sarcoidosis BAL. J Clin Immunol 32(5):1129–1140
Chapman JS (1961) Mycobacterial and mycotic antibodies in sera of patients with sarcoidosis. Results of studies using agar double-diffusion technique. Ann Intern Med 55:918–924
Reid JD, Chiodini RJ (1993) Serologic reactivity against Mycobacterium paratuberculosis antigens in patients with sarcoidosis. Sarcoidosis 10(1):32–35
Drake WP, Dhason MS, Nadaf M, Shepherd BE, Vadivelu S, Hajizadeh R et al (2007) Cellular recognition of Mycobacterium tuberculosis ESAT-6 and KatG peptides in systemic sarcoidosis. Infect Immun 75(1):527–530
Dubaniewicz A, Trzonkowski P, Dubaniewicz-Wybieralska M, Singh M, Mysliwski A (2007) Mycobacterial heat shock protein-induced blood T lymphocytes subsets and cytokine pattern: comparison of sarcoidosis with tuberculosis and healthy controls. Respirology 12(3):346–354
Oswald-Richter KA, Beachboard DC, Zhan X, Gaskill CF, Abraham S, Jenkins C et al (2010) Multiple mycobacterial antigens are targets of the adaptive immune response in pulmonary sarcoidosis. Respir Res 11:161
Agarwal R, Gupta D, Srinivas R, Verma I, Aggarwal AN, Laal S (2012) Analysis of humoral responses to proteins encoded by region of difference 1 of Mycobacterium tuberculosis in sarcoidosis in a high tuberculosis prevalence country. Indian J Med Res 135(6):920–923
Ahmadzai H, Cameron B, Chui JJ, Lloyd A, Wakefield D, Thomas PS (2012) Peripheral blood responses to specific antigens and CD28 in sarcoidosis. Respir Med 106(5):701–709
Inui N, Suda T, Chida K (2008) Use of the QuantiFERON-TB Gold test in Japanese patients with sarcoidosis. Respir Med 102(2):313–315
Horster R, Kirsten D, Gaede KI, Jafari C, Strassburg A, Greinert U et al (2009) Antimycobacterial immune responses in patients with pulmonary sarcoidosis. Clin Respir J 3(4):229–238
Rossman MD, Thompson B, Frederick M, Maliarik M, Iannuzzi MC, Rybicki BA et al (2003) HLA-DRB1*1101: a significant risk factor for sarcoidosis in blacks and whites. Am J Hum Genet 73(4):720–735
Saltini C, Pallante M, Puxeddu E, Contini S, Voorter CE, Drent M et al (2008) M. avium binding to HLA-DR expressed alleles in silico: a model of phenotypic susceptibility to sarcoidosis. Sarcoidosis Vasculitis Diffuse Lung Dis: Off J WASOG / World Assoc Sarcoidosis Other Granulomatous Disord 25(2):100–116
Wiken M, Ostadkarampour M, Eklund A, Willett M, Chen E, Moller D et al (2012) Antigen-specific multifunctional T-cells in sarcoidosis patients with Lofgren’s syndrome. Eur Respir J 40(1):110–121
Koth LL, Solberg OD, Peng JC, Bhakta NR, Nguyen CP, Woodruff PG (2011) Sarcoidosis blood transcriptome reflects lung inflammation and overlaps with tuberculosis. Am J Respir Crit Care Med 184(10):1153–1163
Thillai M, Eberhardt C, Lewin AM, Potiphar L, Hingley-Wilson S, Sridhar S et al (2012) Sarcoidosis and tuberculosis cytokine profiles: indistinguishable in bronchoalveolar lavage but different in blood. PLoS One 7(7):e38083
Maertzdorf J, Weiner J 3rd, Mollenkopf HJ, Bauer T, Prasse A, Muller-Quernheim J et al (2012) Common patterns and disease-related signatures in tuberculosis and sarcoidosis. Proc Natl Acad Sci U S A 109(20):7853–7858
Zhou T, Zhang W, Sweiss NJ, Chen ES, Moller DR, Knox KS et al (2012) Peripheral blood gene expression as a novel genomic biomarker in complicated sarcoidosis. PLoS One 7(9):e44818
Goyal B, Kumar K, Gupta D, Agarwal R, Latawa R, Sheikh JA et al (2014) Utility of B-cell epitopes based peptides of RD1 and RD2 antigens for immunodiagnosis of pulmonary tuberculosis. Diagn Microbiol Infect Dis 78(4):391–397
Homma JY, Abe C, Chosa H, Ueda K, Saegusa J, Nakayama M et al (1978) Bacteriological investigation on biopsy specimens from patients with sarcoidosis. Jpn J Exp Med 48(3):251–255
Eishi Y, Suga M, Ishige I, Kobayashi D, Yamada T, Takemura T et al (2002) Quantitative analysis of mycobacterial and propionibacterial DNA in lymph nodes of Japanese and European patients with sarcoidosis. J Clin Microbiol 40(1):198–204
Ebe Y, Ikushima S, Yamaguchi T, Kohno K, Azuma A, Sato K et al (2000) Proliferative response of peripheral blood mononuclear cells and levels of antibody to recombinant protein from Propionibacterium acnes DNA expression library in Japanese patients with sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 17(3):256–265
Nishiwaki T, Yoneyama H, Eishi Y, Matsuo N, Tatsumi K, Kimura H et al (2004) Indigenous pulmonary Propionibacterium acnes primes the host in the development of sarcoid-like pulmonary granulomatosis in mice. Am J Pathol 165(2):631–639
McCaskill JG, Chason KD, Hua X, Neuringer IP, Ghio AJ, Funkhouser WK et al (2006) Pulmonary immune responses to Propionibacterium acnes in C57BL/6 and BALB/c mice. Am J Respir Cell Mol Biol 35(3):347–356
Ishige I, Eishi Y, Takemura T, Kobayashi I, Nakata K, Tanaka I et al (2005) Propionibacterium acnes is the most common bacterium commensal in peripheral lung tissue and mediastinal lymph nodes from subjects without sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 22(1):33–42
Eishi Y (2013) Etiologic link between sarcoidosis and Propionibacterium acnes. Respir Investig 51(2):56–68
Fretzayas A, Moustaki M, Priftis KN, Yiallouros P, Paschalidou M, Nicolaidou P (2011) Bilateral hilar lymphadenopathy due to Chlamydia pneumoniae infection. Pediatr Pulmonol 46(10):1038–1040
Yano S, Kobayashi K, Ikeda T, Kadowaki T, Wakabayashi K, Kimura M et al (2012) Sarcoid-like reaction in Cryptococcus neoformans infection. BMJ Case Rep 2012
Mathur P, Zurlo JJ, Crook TJ (2014) The intricate relationship of histoplasmosis and sarcoidosis: a case report. J Med Case Rep 8:235
Yang DJ, Krishnan RS, Guillen DR, Schmiege LM 3rd, Leis PF, Hsu S (2006) Disseminated sporotrichosis mimicking sarcoidosis. Int J Dermatol 45(4):450–453
Lebbe C, Agbalika F, Flageul B, Pellet C, Rybojad M, Cordoliani F et al (1999) No evidence for a role of human herpesvirus type 8 in sarcoidosis: molecular and serological analysis. Br J Dermatol 141(3):492–496
Biberfeld P, Petren AL, Eklund A, Lindemalm C, Barkhem T, Ekman M et al (1988) Human herpesvirus-6 (HHV-6, HBLV) in sarcoidosis and lymphoproliferative disorders. J Virol Methods 21(1–4):49–59
Di Alberti L, Piattelli A, Artese L, Favia G, Patel S, Saunders N et al (1997) Human herpesvirus 8 variants in sarcoid tissues. Lancet 350(9092):1655–1661
McKee DH, Young AC, Haeney M (2005) Sarcoidosis and HTLV-1 infection. J Clin Pathol 58(9):996–997
Rottoli P, Bianchi Bandinelli ML, Rottoli L, Zazzi M, Panzardi G, Valensin PE (1990) Sarcoidosis and infections by human lymphotropic viruses. Sarcoidosis 7(1):31–33
Sadikot RT, Dore P, Arnold AG (2001) Sarcoidosis and opportunistic infections. South Med J 94(1):75–77
Fite E, Fernandez-Figueras MT, Prats R, Vaquero M, Morera J (2006) High prevalence of Mycobacterium tuberculosis DNA in biopsies from sarcoidosis patients from Catalonia, Spain. Respiration 73(1):20–26
Labro MT (2012) Immunomodulation and infection: back to the future. Expert Rev Anti-Infect Ther 10(3):245–247
James DG (1991) Mimics of sarcoidosis. Oro-facial granulomatosis (Melkersson-Rosenthal syndrome). Sarcoidosis 8(2):84
Bachelez H, Senet P, Cadranel J, Kaoukhov A, Dubertret L (2001) The use of tetracyclines for the treatment of sarcoidosis. Arch Dermatol 137(1):69–73
Miyazaki E, Ando M, Fukami T, Nureki S, Eishi Y, Kumamoto T (2008) Minocycline for the treatment of sarcoidosis: is the mechanism of action immunomodulating or antimicrobial effect? Clin Rheumatol 27(9):1195–1197
Drake WP, Oswald-Richter K, Richmond BW, Isom J, Burke VE, Algood H et al (2013) Oral antimycobacterial therapy in chronic cutaneous sarcoidosis: a randomized, single-masked, placebo-controlled study. JAMA Dermatol 149(9):1040–1049
Drake W, Richmond BW, Oswald-Richter K, Yu C, Isom JM, Worrell JA et al (2013) Effects of broad-spectrum antimycobacterial therapy on chronic pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 30(3):201–211
Chen ES, Moller DR (2011) Sarcoidosis—scientific progress and clinical challenges. Nat Rev Rheumatol 7(8):457–467
Cosma CL, Humbert O, Ramakrishnan L (2004) Superinfecting mycobacteria home to established tuberculous granulomas. Nat Immunol 5(8):828–835
Ehrenfeld M, Levartowsky D (1989) Serum amyloid-A protein and sarcoidosis. Isr J Med Sci 25(8):418–420
Rubinstein I, Knecht A, de Beer FC, Baum GL, Pras M (1989) Serum amyloid-A protein concentrations in sarcoidosis. Isr J Med Sci 25(8):461–462
Salazar A, Mana J, Fiol C, Hurtado I, Argimon JM, Pujol R et al (2000) Influence of serum amyloid A on the decrease of high density lipoprotein-cholesterol in active sarcoidosis. Atherosclerosis 152(2):497–502
De Vries J, Rothkrantz-Kos S, van Dieijen-Visser MP, Drent M (2004) The relationship between fatigue and clinical parameters in pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 21(2):127–136
Bargagli E, Magi B, Olivieri C, Bianchi N, Landi C, Rottoli P (2011) Analysis of serum amyloid A in sarcoidosis patients. Respir Med 105(5):775–780
Uhlar CM, Whitehead AS (1999) Serum amyloid A, the major vertebrate acute-phase reactant. Eur J Biochem 265(2):501–523
Upragarin N, Landman WJ, Gaastra W, Gruys E (2005) Extrahepatic production of acute phase serum amyloid A. Histol Histopathol 20(4):1295–1307
Eklund KK, Niemi K, Kovanen PT (2012) Immune functions of serum amyloid A. Crit Rev Immunol 32(4):335–348
Song C, Hsu K, Yamen E, Yan W, Fock J, Witting PK et al (2009) Serum amyloid A induction of cytokines in monocytes/macrophages and lymphocytes. Atherosclerosis 207(2):374–383
Migita K, Koga T, Torigoshi T, Motokawa S, Maeda Y, Jiuchi Y et al (2010) Induction of interleukin-23 p19 by serum amyloid A (SAA) in rheumatoid synoviocytes. Clin Exp Immunol 162(2):244–250
Lane AP, Truong-Tran QA, Myers A, Bickel C, Schleimer RP (2006) Serum amyloid A, properdin, complement 3, and toll-like receptors are expressed locally in human sinonasal tissue. Am J Rhinol 20(1):117–123
Sjoholm K, Palming J, Olofsson LE, Gummesson A, Svensson PA, Lystig TC et al (2005) A microarray search for genes predominantly expressed in human omental adipocytes: adipose tissue as a major production site of serum amyloid A. J Clin Endocrinol Metab 90(4):2233–2239
Shah C, Hari-Dass R, Raynes JG (2006) Serum amyloid A is an innate immune opsonin for Gram-negative bacteria. Blood 108(5):1751–1757
Ham D, Caouras V, Radzioch D, Gervais F (1997) Degradation of amyloid A precursor protein SAA by macrophage cell lines obtained from amyloid resistant and susceptible strains of mice. Scand J Immunol 45(4):354–360
Ostadkarampour M, Eklund A, Moller D, Glader P, Hoglund CO, Linden A et al (2014) Higher levels of IL-17 and antigen-specific IL-17 responses in pulmonary sarcoidosis patients with Lofgren’s syndrome. Clin Exp Immunol
Conflict of Interest
Edward Chen and David Moller declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chen, E.S., Moller, D.R. Etiologies of Sarcoidosis. Clinic Rev Allerg Immunol 49, 6–18 (2015). https://doi.org/10.1007/s12016-015-8481-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-015-8481-z