Abstract
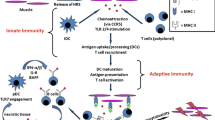
Polymyositis represents an autoimmune disease in which T cells mediate destruction of muscle cells. Although the precise trigger(s) for this process remain unknown, distinct clinical subsets exist that are characterized by antibodies directed against specific nuclear and cytoplasmic antigens including Jo-1 (histidyl-transfer RNA synthetase). Coupled with a range of genetic and histomorphologic data, the stereotypical serologic response suggests that antigen-specific T cells directed against Jo-1 can promote T cell-mediated cytolysis of muscle cells as well as anti-Jo-1 antibody formation in selected patients with polymyositis. Beyond a previously developed animal model that has demonstrated the capacity of Jo-1 to promote humoral and cell-mediated immune responses leading to myositis, recent studies have revealed the existence of Jo-1-specific T cells in the peripheral blood of patients with Jo-1 antibody-positive polymyositis. Even more striking, investigators have discovered that Jo-1 can serve as a chemokine for immature dendritic cells and T lymphocytes. Collectively, these findings suggest a mechanism by which Jo-1 can bridge the innate and adaptive immune responses, leading to the breakdown of tolerance and autoimmune destruction of muscle.
Similar content being viewed by others
References and Recommended Reading
Oddis CV: Idiopathic inflammatory myopathies. In Clinical Disorders of Skeletal Muscle. Edited by Wortmann RL. Philadelphia: Lippincott-Raven Press; 1999:45–87.
Dalakas MC, Sivakumar K: The immunopathologic and inflammatory differences between dermatomyositis, polymyositis and sporadic inclusion body myositis. Curr Opin Neurol 1996, 9:235–239.
Kissel JT, Mendell JR, Rammohan KW: Microvascular deposition of complement membrane attack complex in dermatomyositis. N Engl J Med 1986, 314:329–334.
Emslie-Smith AM, Engel AG: Microvascular changes in early and advanced dermatomyositis: a quantitative study. Ann Neurol 1990, 27:343–356.
Targoff IN: Humoral immunity in polymyositis/dermatomyositis. J Invest Dermatol 1993, 100:116S-123S.
Cherin P, Herson S, Crevon MC, et al.: Mechanisms of lysis by activated cytotoxic cells expressing perforin and granzyme-B genes and the protein TIA-1 in muscle biopsies of myositis. J Rheum 1996, 23:1135–1142.
Behrens L, Bender A, Johnson MA, Hohlfeld R: Cytotoxic mechanisms in inflammatory myopathies: co-expression of Fas and protective Bcl-2 in muscle fibers and inflammatory cells. Brain 1997, 120:929–938.
Sugiura T, Murakawa Y, Nagai A, et al.: Fas and Fas ligand interaction induces apoptosis in inflammatory myopathies. Arthritis Rheum 1999, 42:291–298.
Choi YC, Dalakas MC: Expression of matrix metalloproteinases in the muscle of patients with inflammatory myopathies. Neurology 2000, 54:65–70.
Tateyama M, Nagano I, Yoshioka M, et al.: Expression of tumor necrosis factor-alpha in muscles of polymyositis. J Neurol Sci 1997, 146:45–51.
Kuru S, Inukai A, Liang Y, et al.: Tumor necrosis facor-alpha expression in muscles of polymyositis and dermatomyositis. Acta Neuropathol 2000, 99:585–588.
Miller FW, Waite KA, Biswas T, Plotz PH: The role of an autoantigen, histidyl-tRNA synthetase, in the induction and maintenance of autoimmunity. Proc Natl Acad Sci U S A 1990, 87:9933–9937. Through analysis of the anti-Jo-1 antibody response in a patient with myositis at the onset of disease, this study demonstrates the process of affinity maturation indicative of an immune response driven by human histidyl-transfer RNA synthetase (Jo-1) rather than crossreactivity to foreign proteins.
Miller FW, Twitty SA, Biswas T, Plotz PH: Origin and regulation of a disease-specific autoantibody response. J Clin Invest 1990, 85:468–475.
Raben N, Nichols R, Dohlman J, et al.: A motif in human histidyl-tRNA synthetase which is shared among several aminoacyl-tRNA synthetases is a coiled-coil that is essential for enzymatic activity and contains the major autoantigenic epitope. J Biol Chem 1994, 269:24277–24283. Although from 1994, this work localized the major B cell epitope recognized by anti-Jo-1 human sera to the amino-terminal 47 amino acids of histidyl-transfer RNA synthetase. Later work by Casciola- Rosen et al. [36] demonstrated a granzyme B cleavage site at amino acid 48 of histidyl-transfer RNA synthetase.
Martin A, Shulman MJ, Tsui FW: Epitope studies indicate that histidyl-tRNA synthetase is a stimulating antigen in idiopathic myositis. FASEB J 1995, 9:1226–1233.
Craft J, Fatenejad S: Self antigens and epitope spreading in systemic autoimmunity. Arthritis Rheum 1997, 40:1374–1382.
Kita H, Matsumura S, He XS, et al.: Quantitative and functional analysis of PDC-E2-specific autoreactive cytotoxic T lymphocytes in primary biliary cirrhosis. J Clin Invest 2002, 109:1231–1240. Using pyruvate dehydrogenase complex-E2 (PDC-E2) peptide-pulsed dendritic cells, the investigators were able to amplify peptide-specific CD8+ T cells from peripheral blood as well as liver-infiltrating lymphocyte populations in patients with primary biliary cirrhosis. Because CD4+ T cells and B cells from these patients recognize the same antigen (PDC-E2), this human disease provides a paradigm for the proposed role of Jo-1 in directing the humoral and cell-mediated immune responses of PM.
Akbar S, Yamamoto K, Miyaki H, et al.: Peripheral blood T-cell responses to pyruvate dehydrogenase complex in primary biliary cirrhosis: role of antigen-presenting dendritic cells. Eur J Clin Invest 2001, 31:639–646.
Yeaman S, Kirby J, Jones D: Autoreactive responses to pyruvate dehydrogenase complex in the pathogenesis of primary biliary cirrhosis. Immunol Rev 2000, 174:238–249.
Palmer J, Diamond A, Yeaman S, et al.: T cell responses to the putative dominant autoepitope in primary biliary cirrhosis (PBC). Clin Exp Immunol 1999, 116:133–139.
Blechynden LM, Lawson MM, Tabarias H, et al.: Myositis induced by naked DNA immunization with the gene for histidyl-tRNA synthetase. Hum Gene Ther 1997, 8:1469–1480. Through DNA immunization of mice with the histidyl-transfer RNA synthetase gene, the authors were able to demonstrate Jo-1 autoantibody formation as well as localized myositis. This animal model therefore supports a direct role for Jo-1 in the pathogenesis of myositis.
Nagaraju K, Raben N, Loeffler L, et al.: Conditional upregulation of MHC class I in skeletal muscle leads to self-sustaining autoimmune myositis and myositis-specific antibodies. Proc Natl Acad Sci USA 2000, 97:9209–9214. This work describes a murine model of spontaneous PM resulting from inducible, tissue-specific upregulation of MHC class I expression. Characterized by predominant macrophage or monocyte infiltration of muscle as well as autoantibody formation, this experimental model suggests a potential mechanism for the breadown of tolerance leading to inflammatory muscle disease in humans.
O’Hanlon TP, Dalakas MC, Plotz PH, Miller FW: Predominant TCR-ab variable and joining gene expression by muscleinfiltrating lymphocytes in the idiopathic inflammatory myopathies. J Immunol 1994, 152:2569–2576.
Bender A, Ernst N, Iglesias A, et al.: T cell receptor repertoire in polymyositis: clonal expansion of autoaggressive CD8+ T cells. J Exp Med 1995, 181:1863–1868. By demonstrating oligoclonal T cell expansion in muscle biopsy specimens from patients with PM, this analysis provides compelling evidence that this disease process is antigen driven.
Kuwana M, Medsger TA, Jr., Wright TM: T cell proliferative response induced by DNA topoisomerase I in patients with systemic sclerosis and healthy donors. J Clin Invest 1995, 6:586–596.
Kuwana M, Medsger TA Jr., Wright TM: Highly restricted TCRalpha/ beta usage by autoreactive human T cell clones specific for DNA topoisomerase I. J Immunol 1997, 158:485–491.
Kuwana M, Medsger TA, Jr., Wright TM: Analysis of soluble and cell surface factors regulating anti-DNA topoisomerase I autoantibody production demonstrates synergy between Th1 and Th2 autoreactive T cells. J Immunol 2000, 164:6138–6146.
Hattori N, Kuwana M, Kaburaki J, et al.: T cells that are autoreactive to beta-2-glycoprotein I in patients with antiphospholipid syndrome and healthy individuals. Arthritis Rheum 2000, 43:65–75.
Kuwana M, Kaburaki J, Ikeda Y: Autoreactive T cells to platelet GPIIb-IIIa in immune thrombocytopenic purpura. J Clin Invest 1998, 102:1393–1402.
Goldstein R, Duvic M, Targoff IN, et al.: HLA-D region genes associated with autoantibody responses to histidyl-tRNA synthetase (Jo-1) and other translation-related factors in myositis. Arthritis Rheum 1990, 33:1240–1248.
Arnett FC, Targoff IN, Mimori T, et al.: Interrelationship of major histocompatibility complex class II alleles and autoantibodies in four ethnic groups with various forms of myositis. Arthritis Rheum 1996, 39:1507–1518.
Garlep MJ: Genetics of the idiopathic inflammatory myopathies. Curr Opin Rheumatol 1996, 8:514–520.
Ascherman D, Oriss T, Oddis CV, Wright TM: Critical requirement for professional APCs in eliciting T cell responses to novel fragments of histidyl-tRNA synthetase (Jo-1) in Jo-1 antibody-positive polymyositis. J Immunol 2002, 169:7127–7134. This work represents the first demonstration of Jo-1-specific T cell responses in patients with Jo-1 antibody PM as well as healthy control individuals. Analysis of proliferative responses to fragments of Jo-1 presented by dendritic cells reveals an immudominant T cell epitope contained within the amino terminal 150 amino acids of this protein.
O’Hanlon TP, Miller FW: Genomic organization, transcriptional mapping, and evolutionary implications of the human bi-directional histidyl-tRNA synthetase locus (HARS/ HARSL). Biochem Biophys Res Commun 1995, 294:609–614.
Rosen A, Casciola-Rosen L: Autoantigens as substrates for apoptotic proteases: implications for the pathogenesis of systemic autoimmune disease. Cell Death Differ 1999, 6:6–12. This review describes the potential autoantigenic role of “cryptic” epitopes generated through cleavage of selected proteins by proteases activated during apoptosis.
Casciola-Rosen L, Andrade F, Ulanet D, et al.: Cleavage by granzyme B is strongly predictive of autoantigen status: implications for the initiation of autoimmunity. J Exp Med 1999, 190:815–826. This work provides strong evidence that seemingly disparate autoantigens share the common feature of granzyme B-cleavability. Elaborated during cytotoxic lymphocyte-mediated cell death, this enzyme is capable of generating novel epitopes that may contribute to the breakdown of tolerance and generation of autoimmunity.
Utz P, Anderson P: Postranslational protein modifications, apoptosis, and the bypass of tolerance to autoantigens. Arthritis Rheum 1998, 41:1152–1160. This review nicely summarizes how alterations in antigenic structure during apoptosis can result in tolerance breakdown.
Wakasugi K, Schimmel P: Two distinct cytokines released from a human aminoacyl-tRNA synthetase. Science 1999, 284:147–151. In a novel set of experiments suggested by analysis of the domain structure of tyrosyl-transfer RNA synthetase, these authors demonstrate functional cytokine properties for amino- and carboxy-terminal fragments of this enzyme.
Wakasugi K, Slike B, Hood J, et al.: A human aminoacyl-tRNA synthetase as a regulator of angiogenesis. Proc Natl Acad Sci U S A 2002, 99:173–177.
Nagaraju K: Immunological capabilities of skeletal muscle cells. Acta Physiol Scand 2001, 171:215–223.
Howard OMZ, Dong HF, Yang D, et al.: Histidyl-tRNA synthetase and asparaginyl-tRNA synthetase, autoantigens in myositis, activate chemokine receptors on T lymphocytes and immature dendritic cells. J Exp Med 2002, 196:781–791. This study extends earlier work regarding non-enzymatic biologic functions of aminoacyl-transfer RNA synthetases, demonstrating chemokine properties of Jo-1. Because Jo-1 interacts with chemokine receptors on immature dendritic cells as well as T cells, the authors speculate that Jo-1 may be able to link the innate and adaptive immune response in PM.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ascherman, D.P. The role of jo-1 in the immunopathogenesis of polymyositis: Current hypotheses. Curr Rheumatol Rep 5, 425–430 (2003). https://doi.org/10.1007/s11926-003-0052-2
Issue Date:
DOI: https://doi.org/10.1007/s11926-003-0052-2