Abstract
Introduction
Population modelling using mixed effects models provides a means to study variability in paediatric drug responses among individuals representative of those in whom the drug will be used clinically.
Discussions
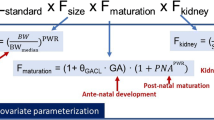
Explanatory covariates explain the predictable part of the between-individual variability. Growth and development are two major aspects of children not seen in adults. These aspects can be investigated by using size and age as covariates. Problems attributable to co-linearity can be approached by using size as the first covariate. Size standardisation is achieved using allometric scaling, a mechanistic approach that has a strong theoretical and empirical basis. Age is used to describe the maturation of clearance. The quantitative models (linear, exponential, first-order, variable slope sigmoidal) used to describe this maturation process vary depending on the span of the ages under investigation. Measures of response are not always straightforward and can be more difficult to quantify in children.
Conclusion
Covariate investigation in children is improving the understanding of developmental aspects of drug disposition and effects in the paediatric population, ultimately leading to more effective use of medications.





Similar content being viewed by others
Abbreviations
- BMR:
-
Basal metabolic rate
- BSA:
-
Body surface area
- CL:
-
Clearance
- CLcr:
-
Creatinine clearance
- CPR:
-
Creatinine production rate
- GFR:
-
Glomerular filtration rate ka; absorption rate constant
- ln:
-
Natural logarithm
- NONMEM:
-
Nonlinear mixed effects model
- PD:
-
Pharmacodynamics
- PK:
-
Pharmacokinetics
- PMA:
-
Postmenstrual age
- PNA:
-
Postnatal age
- PWR:
-
Allometric power exponent
- Tabs:
-
Absorption half time
- TDM:
-
Therapeutic drug monitoring
- V:
-
Volume of distribution
References
Allegaert K, Anderson BJ, Cossey V, Holford NH (2006) Limited predictability of amikacin clearance in extreme premature neonates at birth. Br J Clin Pharmacol 61(1):39–48
Allegaert K, Anderson BJ, Verbesselt R, Debeer A, de Hoon J, Devlieger H, van den Anker JN, Tibboel D (2005) Tramadol disposition in the very young: an attempt to assess in vivo cytochrome P-450 2D6 activity. Br J Anaesth 95(2):231–239
Anderson BJ (2005) Phenytoin elimination in a child during hypothermia for traumatic brain injury. Paediat Perinat Drug Ther 6(3):133–138
Anderson BJ, Allegaert K, van den Anker JN, Cossey V, Holford NHG (2006) Vancomycin pharmacokinetics in preterm neonates and the prediction of adult clearance. Brit J Clin Pharmacol (in press)
Anderson BJ, Meakin GH (2002) Scaling for size: some implications for paediatric anaesthesia dosing. Paediatr Anaesth 12(3):205–219
Anderson BJ, Pons G, Autret-Leca E, Allegaert K, Boccard E (2005) Pediatric intravenous paracetamol (propacetamol) pharmacokinetics: a population analysis. Paediatr Anaesth 15(4):282–292
Anderson BJ, Ralph CJ, Stewart AW, Barber C, Holford NH (2000) The dose–effect relationship for morphine and vomiting after day-stay tonsillectomy in children. Anaesth Intensive Care 28(2):155–160
Anderson BJ, van Lingen RA, Hansen TG, Lin YC, Holford NH (2002) Acetaminophen developmental pharmacokinetics in premature neonates and infants: a pooled population analysis. Anesthesiology 96(6):1336–1345
Anderson BJ, Woollard GA, Holford NH (2000) A model for size and age changes in the pharmacokinetics of paracetamol in neonates, infants and children. Br J Clin Pharmacol 50(2):125–134
Anderson BJ, Woollard GA, Holford NH (2001) Acetaminophen analgesia in children: placebo effect and pain resolution after tonsillectomy. Eur J Clin Pharmacol 57(8):559–569
Arant BS Jr (1978) Developmental patterns of renal functional maturation compared in the human neonate. J Pediatr 92(5):705–712
Beal S, Sheiner L (1980) The NONMEM system. Am Stat 34:118–119
Beal SL (1991) Computing initial estimates with mixed effects models: a general method of moments. Biometrika 78(1):217–220
Bergstein JM (2000) Introduction to glomerular diseases. In: Behrman RE, Kliegman RM, Jenson HB (ed) Nelson textbook of pediatrics, 16th edn. WB Saunders, Philadelphia, Pennsylvania, pp 1574–1575
Bonate PL (1999) The effect of collinearity on parameter estimates in nonlinear mixed effect models. Pharm Res 16(5):709–717
Booker PD, Taylor C, Saba G (1996) Perioperative changes in alpha 1-acid glycoprotein concentrations in infants undergoing major surgery. Br J Anaesth 76(3):365–368
Bouwmeester NJ, Anderson BJ, Tibboel D, Holford NH (2004) Developmental pharmacokinetics of morphine and its metabolites in neonates, infants and young children. Br J Anaesth 92(2):208–217
Boxenbaum H, DiLea C (1995) First-time-in-human dose selection: allometric thoughts and perspectives. J Clin Pharmacol 35(10):957–966
Brown RD, Kearns GL, Wilson JT (1998) Integrated pharmacokinetic–pharmacodynamic model for acetaminophen, ibuprofen, and placebo antipyresis in children. J Pharmacokinet Biopharm 26(5):559–579
Capparelli EV, Englund JA, Connor JD, Spector SA, McKinney RE, Palumbo P, Baker CJ (2003) Population pharmacokinetics and pharmacodynamics of zidovudine in HIV-infected infants and children. J Clin Pharmacol 43(2):133–140
Chugani DC, Muzik O, Juhasz C, Janisse JJ, Ager J, Chugani HT (2001) Postnatal maturation of human GABAA receptors measured with positron emission tomography. Ann Neurol 49(5):618–626
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16(1):31–41
Cole M, Price L, Parry A, Keir MJ, Pearson AD, Boddy AV, Veal GJ (2004) Estimation of glomerular filtration rate in paediatric cancer patients using 51CR-EDTA population pharmacokinetics. Br J Cancer 90(1):60–64
Crawford JD, Terry ME, Rourke GM (1950) Simplification of drug dosage calculation by application of the surface area principle. Pediatrics 5(5):783–790
Dawson WT (1940) Relations between age and weight and dosages of drugs. Ann Intern Med 13:1594–1613
Du Bois D, Du Bois EF (1916) Clinical calorimetry: tenth paper. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med 17:863–871
Friis-Hansen B (1961) Body water compartments in children: changes during growth and related changes in body composition. Pediatrics 28:169–181
Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL (2001) Effects of size and temperature on metabolic rate. Science 293(5538):2248–2251
Grimsley C, Thomson AH (1999) Pharmacokinetics and dose requirements of vancomycin in neonates. Arch Dis Child Fetal Neonatal Ed 81(3):F221–F227
Guignard JP, Drukker A (1999) Why do newborn infants have a high plasma creatinine? Pediatrics 103(4):e49
Hines RN, McCarver DG (2002) The ontogeny of human drug-metabolizing enzymes: phase I oxidative enzymes. J Pharmacol Exp Ther 300(2):355–360
Holford NHG (1996) A size standard for pharmacokinetics. Clin Pharmacokinet 30(5):329–332
Hull CJ, Van Beem HB, McLeod K, Sibbald A, Watson MJ (1978) A pharmacodynamic model for pancuronium. Br J Anaesth 50(11):1113–1123
Kan RE, Hughes SC, Rosen MA, Kessin C, Preston PG, Lobo EP (1998) Intravenous remifentanil: placental transfer, maternal and neonatal effects. Anesthesiology 88(6):1467–1474
Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE (2003) Developmental pharmacology—drug disposition, action, and therapy in infants and children. N Engl J Med 349(12):1157–1167
Kimura T, Sunakawa K, Matsuura N, Kubo H, Shimada S, Yago K (2004) Population pharmacokinetics of arbekacin, vancomycin, and panipenem in neonates. Antimicrob Agents Chemother 48(4):1159–1167
Kleiber M (1961) The fire of life: an introduction to animal energetics. Wiley, New York
Knibbe CA, Zuideveld KP, Aarts LP, Kuks PF, Danhof M (2005) Allometric relationships between the pharmacokinetics of propofol in rats, children and adults. Br J Clin Pharmacol 59(6):705–711
Koukouritaki SB, Manro JR, Marsh SA, Stevens JC, Rettie AE, McCarver DG, Hines RN (2004) Developmental expression of human hepatic CYP2C9 and CYP2C19. J Pharmacol Exp Ther 308(3):965–974, Epub 2003 Nov 21
Leger F, Bouissou F, Coulais Y, Tafani M, Chatelut E (2002) Estimation of glomerular filtration rate in children. Pediatr Nephrol 17(11):903–907
Lynn AM, Nespeca MK, Opheim KE, Slattery JT (1993) Respiratory effects of intravenous morphine infusions in neonates, infants, and children after cardiac surgery. Anesth Analg 77(4):695–701
Marshall JD, Kearns GL (1999) Developmental pharmacodynamics of cyclosporine. Clin Pharmacol Ther 66(1):66–75
Matthews I, Kirkpatrick C, Holford N (2004) Quantitative justification for target concentration intervention—parameter variability and predictive performance using population pharmacokinetic models for aminoglycosides. Br J Clin Pharmacol 58(1):8–19
Mitchell D, Strydon NB, van Graun CH, van der Walt WH (1971) Human surface area: comparison of the Du Bois formula with direct photometric measurement. Pfugers Arch 325(2):188–190
Paap CM, Nahata MC (1995) Prospective evaluation of ten methods for estimating creatinine clearance in children with varying degrees of renal dysfunction. J Clin Pharm Ther 20(2):67–73
Pacifici GM, Franchi M, Giuliani L, Rane A (1989) Development of the glucuronyltransferase and sulphotransferase towards 2-naphthol in human fetus. Dev Pharmacol Ther 14(2):108–114
Pacifici GM, Sawe J, Kager L, Rane A (1982) Morphine glucuronidation in human fetal and adult liver. Eur J Clin Pharmacol 22(6):553–558
Peeters MY, Prins SA, Knibbe CA, Dejongh J, van Schaik RH, van Dijk M, van der Heiden IP, Tibboel D, Danhof M (2006) Propofol pharmacokinetics and pharmacodynamics for depth of sedation in nonventilated infants after major craniofacial surgery. Anesthesiology 104(3):466–474
Peters HP (1983) Physiological correlates of size. In: Beck E, Birks HJB, Conner EF (eds) The ecological implications of body size. Cambridge University Press, Cambridge, UK, pp 48–53
Rajagopalan P, Gastonguay MR (2003) Population pharmacokinetics of ciprofloxacin in pediatric patients. J Clin Pharmacol 43(7):698–710
Ross AK, Davis PJ, Dear Gd GL, Ginsberg B, McGowan FX, Stiller RD, Henson LG, Huffman C, Muir KT (2001) Pharmacokinetics of remifentanil in anesthetized pediatric patients undergoing elective surgery or diagnostic procedures. Anesth Analg 93(6):1393–1401
Schmidt-Nielsen K (1984) Scaling: why is animal size so important? Cambridge University Press, Cambridge, UK
Schuttler J, Ihmsen H (2000) Population pharmacokinetics of propofol: a multicenter study. Anesthesiology 92(3):727–738
Schwartz GJ, Feld LG, Langford DJ (1984) A simple estimate of glomerular filtration rate in full-term infants during the first year of life. J Pediatr 104(6):849–854
Schwartz GJ, Gauthier B (1985) A simple estimate of glomerular filtration rate in adolescent boys. J Pediatr 106(3):522–526
Sheiner LB, Stanski DR, Vozeh S, Miller RD, Ham J (1979) Simultaneous modeling of pharmacokinetics and pharmacodynamics: application to D-tubocurarine. Clin Pharmacol Ther 25(3):358–371
Sonntag J, Prankel B, Waltz S (1996) Serum creatinine concentration, urinary creatinine excretion and creatinine clearance during the first 9 weeks in preterm infants with a birth weight below 1500 g. Eur J Pediatr 155(9):815–819
Tenenbein M (2000) Why young children are resistant to acetaminophen poisoning. J Pediatr 137(6):891–892
van der Heijden AJ, Grose WF, Ambagtsheer JJ, Provoost AP, Wolff ED, Sauer PJ (1988) Glomerular filtration rate in the preterm infant: the relation to gestational and postnatal age. Eur J Pediatr 148(1):24–28
van der Marel CD, Anderson BJ, Romsing J, Jacqz-Aigrain E, Tibboel D (2004) Diclofenac and metabolite pharmacokinetics in children. Paediatr Anaesth 14(6):443–451
van Dijk M, Koot HM, Saad HH, Tibboel D, Passchier J (2002) Observational visual analog scale in pediatric pain assessment: useful tool or good riddance? Clin J Pain 18(5):310–316
van Dijk M, Peters JW, Bouwmeester NJ, Tibboel D (2002) Are postoperative pain instruments useful for specific groups of vulnerable infants? Clin Perinatol 29(3):469–491
van Dijk M, Simons SH, Tibboel D (2004) Pain assessment in neonates. Paed Perinatal Drug Ther 6(2):97–103
Way WL, Costley EC, Way EL (1965) Respiratory sensitivity of the newborn infant to meperidine and morphine. Clin Pharmacol Ther 6:454–461
West GB, Brown JH, Enquist BJ (1997) A general model for the origin of allometric scaling laws in biology. Science 276(5309):122–126
West GB, Brown JH, Enquist BJ (1999) The fourth dimension of life: fractal geometry and allometric scaling of organisms. Science 284(5420):1677–1679
Yukawa E, Satou M, Nonaka T, Yukawa M, Ohdo S, Higuchi S, Kuroda T, Goto Y (2002) Pharmacoepidemiologic investigation of clonazepam relative clearance by mixed-effect modeling using routine clinical pharmacokinetic data in Japanese patients. J Clin Pharmacol 42(1):81–88
Acknowledgement
The clinical research of K. Allegaert is supported by the Fund for Scientific Research, Flanders (Belgium) by a Clinical Doctoral Grant (A 6/5–KV–G 1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anderson, B.J., Allegaert, K. & Holford, N.H.G. Population clinical pharmacology of children: modelling covariate effects. Eur J Pediatr 165, 819–829 (2006). https://doi.org/10.1007/s00431-006-0189-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-006-0189-x