Abstract
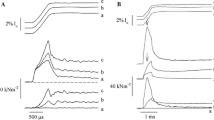
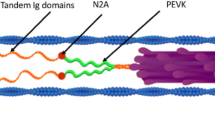
The effect of titin-based passive tension on Ca2+ sensitivity of active tension and interfilament lattice spacing was studied in skinned rat ventricular trabeculae by measuring the sarcomere length (SL)-dependent change in Ca2+ sensitivity and performing small angle X-ray diffraction studies. To vary passive tension, preparations were treated with trypsin at a low concentration (0.31 μg/ml) for a short period (13 min) at 20°C, that resulted in ~40% degradation of the I-band region of titin, with a minimal effect on A-band titin. We found that the effect of trypsin on titin-based passive tension was significantly more pronounced immediately after stretch than at steady state, 30 min after stretch (i.e., trypsin has a greater effect on viscosity than on elasticity of passive cardiac muscle). Ca2+ sensitivity was decreased by trypsin treatment at SL 2.25 μm, but not at SL 1.9 μm, resulting in marked attenuation of the SL-dependent increase in Ca2+ sensitivity. The SL-dependent change in Ca2+ sensitivity was significantly correlated with titin-based passive tension. Small-angle X-ray diffraction experiments revealed that the lattice spacing expands after trypsin treatment, especially at SL 2.25 μm, providing an inverse linear relationship between the lattice spacing and Ca2+ sensitivity. These results support the view that titin-based passive tension promotes actomyosin interaction and that the mechanism includes interfilament lattice spacing modulation.



Similar content being viewed by others
References
Allen DG, Kentish JC (1985) The cellular basis for length-tension relation in cardiac muscle. J Mol Cell Cardiol 17: 821–840
Astier C, Labbe J, Roustan C, Benyamin Y (1993) Effects of different enzymic treatments on the release of titin fragments from rabbit skeletal myofibrils. Biochem J 290:731–734
Bang ML, Centner T, Fornoff F, Geach AJ, Gotthardt M, McNabb M, Witt CC, Labeit D, Gregorio CC, Granzier H, Labeit S (2001) The complete gene sequence of titin, expression of an unusual approximately 700-kDa titin isoform, and its interaction with obscurin identify a novel Z-line to I-band linking system. Circ Res 89:1065–1072
Cazorla O, Vassort G, Garnier D, Le Guennec JY (1999) Length modulation of active force in rat cardiac myocytes: is titin the sensor? J Mol Cell Cardiol 31: 1215–1227
Cazorla O, Freiburg A, Helmes M, Centner T, McNabb M, Wu Y, Trombitas K, Labeit S, Granzier H (2000) Differential expression of cardiac titin isoforms and modulation of cellular stiffness. Circ Res 86:59–67
Cazorla O, Wu Y, Irving TC, Granzier H (2001) Titin-based modulation of calcium sensitivity of active tension in mouse skinned cardiac myocytes. Circ Res 88: 1028–1035
Dobesh DP, Konhilas JP, de Tombe PP (2002) Cooperative activation in cardiac muscle: impact of sarcomere length. Am J Physiol Heart 282:H1055–H1062
Fitzsimons DP, Moss RL (1998) Strong binding of myosin modulates length-dependent Ca2+ activation of rat ventricular myocytes. Circ Res 83: 602–607
Fuchs F, Wang YP (1996) Sarcomere length versus interfilament lspacing as determinants of cardiac myofilament Ca2+ sensitivity and Ca2+ binding. J Mol Cell Cardiol 28:1375–1383
Fukuda N, Granzier H (2004) Role of the giant elastic protein titin in the Frank-Starling mechanism of the heart. Curr Vasc Pharmacol 2:135–139
Fukuda N, Kajiwara H, Ishiwata S, Kurihara S (2000) Effects of MgADP on length dependence of tension generation in skinned rat cardiac muscle. Circ Res 86:e1–e6
Fukuda N, Sasaki D, Ishiwata S, Kurihara S (2001) Length dependence of tension generation in rat skinned cardiac muscle: role of titin in the Frank-Starling mechanism of the heart. Circulation 104:1639–1645
Fukuda N, Wu Y, Irving TC, Granzier H (2003) Titin isoform variance and length dependence of activation in skinned bovine cardiac muscle. J Physiol (Lond) 553:147–154
Gordon AM, Huxley AF, Julian FJ (1966) The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol (Lond) 184:170–192
Granzier H, Irving TC (1995) Passive tension in cardiac muscle: contribution of collagen, titin, microtubles, and intermediate filaments. Biophys J 68:1027–1044
Granzier HL, Wang K (1993) Gel electrophoresis of giant proteins: solubilization and silver-staining of titin and nebulin from single muscle fiber segments. Electrophoresis 14:56–64
Haase H, Pagel I, Khalina Y, Zacharzowsky U, Person V, Lutsch G, Petzhold D, Kott M, Schaper J, Morano I (2004) The carboxyl-terminal ahnak domain induces actin bundling and stabilizes muscle contraction. FASEB J 18:839–841
Helmes M, Trombitas K, Granzier H (1996) Titin develops restoring force in rat cardiac myocytes. Circ Res 79: 619–626
Helmes M, Lim CC, Liao R, Bharti A, Cui L, Sawyer DB (2003) Titin determines the Frank-Starling relation in early diastole. J Gen Physiol 121:97–110
Irving TC, Konhilas J, Perry D, Fischetti R, de Tombe PP (2000) Myofilament lattice spacing as a function of sarcomere length in isolated rat myocardium. Am J Physiol Heart 279:H2568–H2573
Kentish JC (1986) The effects of inorganic phosphate and creatine phosphate on force production in skinned muscles from rat ventricle. J Physiol (Lond) 370:585–604
Kentish JC, ter Keurs HEDJ, Ricciardi L, Bucx JJJ, Noble MIM (1986) Comparison between the sarcomere length-force relations of intact and skinned trabeculae from rat right ventricle. Influcence of calcium concentrations on these relations. Circ Res 58:755–768
Konhilas JP, Irving TC, de Tombe PP (2002) Myofilament calcium sensitivity in skinned rat cardiac trabeculae: role of interfilament spacing. Circ Res 90:59–65
Konhilas JP, Irving TC, de Tombe PP (2002) Length-dependent activation in three striated muscle types of the rat. J Physiol (Lond) 544:225–236
Konhilas JP, Irving TC, Wolska BM, Jweied EE, Martin AF, Solaro RJ, de Tombe PP (2003) Troponin I in the murine myocardium: influence on length-dependent activation and interfilament spacing. J Physiol (Lond) 547:951–961
Kulke M, Fujita-Becker S, Rostkova E, Neagoe C, Labeit D, Manstein DJ, Gautel M, Linke WA (2001) Interaction between PEVK-titin and actin filaments: origin of a viscous force component in cardiac myofibrils. Circ Res 89:874–881
Labeit S, Gautel M, Lakey A, Trinick J (1992) Towards a molecular understanding of titin. EMBO J 11:1711–1716
Lakatta EG (1987) Starling’s law of the heart is explained by an intimate interaction of muscle length and myofilament calcium activation. J Am Col Cardiol 10:1157–1164
McDonald KS, Moss RL (1995) Osmotic compression of single cardiac myocytes eliminates the reduction in Ca2+ sensitivity of tension at short sarcomere length. Circ Res 77:199–205
Mio Y, Fukuda N, Kusakari Y, Tanifuji Y, Kurihara S (2002) Bupivacaine attenuates contractility by decreasing sensitivity of myofilaments to Ca2+ in rat ventricular muscle. Anesthesiology 97:1168–1177
Moss RL, Fitzsimons DP (2002) Frank-Starling relationship. Long on importance, short on mechanism. Circ Res 90:11–13
Rice JJ, de Tombe PP (2004) Approaches to modeling crossbridges and calcium-dependent activation in cardiac muscle. Prog Biophys Mol Biol 85:179–195
Trombitas K, Granzier H (1997) Actin removal from cardiac myocytes show that near Z-line titin attaches to actin while under tension. Am J Physiol 273:C662–C670
Trombitas K, Jin JP, Granzier H (1995) The mechanically active domain of titin in cardiac muscle. Circ Res 77:856–861
Wu Y, Cazolra O, Labeit D, Labeit S, Granzier H (2000) Changes in titin and collagen underlie diastolic stiffness diversity of cardiac muscle. J Mol Cell Cardiol 32:2151–2162
Yamasaki R, Berri M, Wu Y, Trombitas K, McNabb M, Kellermayer MS, Witt C, Labeit D, Labeit S, Greaser M, Granzier H (2001) Titin-actin interaction in mouse myocardium: passive tension modulation and its regulation by calcium/S100A1. Biophys J 81:2297–2313
Yamasaki R, Wu Y, McNabb M, Greaser M, Labeit S, Granzier H (2002) Protein kinase A phosphorylates titin’s cardiac-specific N2B domain and reduces passive tension in rat cardiac myocytes. Circ Res 90:1181–1188
Acknowledgements
This work is supported by a National Institutes of Health (NIH) Grant HL62881 (to H.G.). N.F. is a recipient of a grant from the Uehara Memorial Foundation (Tokyo, Japan). We would like to thank J. Henry, G.A. Kumar, and A. Joshi for help with X-ray data analysis. Use of the Advanced Photon Source was supported by the US Department of Energy, Basic Energy Sciences, Office of Energy Research, under Contract No. W-31-109-ENG-38. BioCAT is an NIH-supported Research Center (RR08630).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fukuda, N., Wu, Y., Farman, G. et al. Titin-based modulation of active tension and interfilament lattice spacing in skinned rat cardiac muscle. Pflugers Arch - Eur J Physiol 449, 449–457 (2005). https://doi.org/10.1007/s00424-004-1354-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-004-1354-6