Abstract
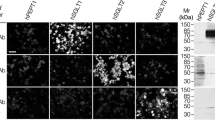
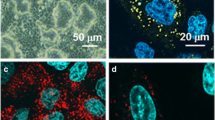
Functional evidence of Na+–glucose cotransport in rat lung has been provided by Basset et al. (J. Physiol. 384:325–345, 1987). By autoradiography [3H]phloridzin binding has been found confined to alveolar epithelial type II cells in mouse and rabbit lungs (Boyd, J. Physiol. 422: 44P, 1990). In this research we checked by immunofluorescence whether Na+–glucose cotransporter (SGLT1) is also expressed in alveolar type I cells. Lungs of anesthetized rats and lambs were fixed by paraformaldehyde, perfused in pulmonary artery, or instilled into a bronchus, respectively. Tissue blocks embedded in paraffin or frozen were sectioned. Two specific anti-SGLT1 antibodies for rat recognizing aminoacid sequence 402–420, and 546–596 were used in both species. Bound primary antibody was detected by secondary antibody conjugated to fluorescein isothiocianate or Texas red, respectively. In some sections cellular nuclei were also stained. In rats alveolar type I cells were identified by fluorescent Erythrina cristagalli lectin. Sections were examined by confocal laser-scanning microscope. Both in rats and lambs alveolar epithelium was stained by either antibody; no labeling occurred in negative controls. Hence, SGLT1 appears to be also expressed in alveolar type I cells. This is functionally relevant because type I cells provide 95–97% of alveolar surface, and SGLT1, besides contributing to removal of lung liquid under some circumstances, keeps low glucose concentration in lining liquid, which is useful to prevent lung infection.




Similar content being viewed by others
References
Baker EH, Wood DM, Brennan AL, Clark N, Baines DL, Philips BJ (2006) Hyperglycaemia and pulmonary infection. Proc Nutr Soc 65:227–235
Bankston PW, Porter GA, Milici AJ, Palade GE (1991) Differential and specific labeling of epithelial and vascular endothelial cells of the rat lung by Lycopersicon esculentum and Griffonia simplicifolia I lectins. Eur J Cell Biol 54:187–195
Basset G, Crone C, Saumon G (1987) Fluid absorption by rat lung in situ: pathways for sodium entry in the luminal membrane of alveolar epithelium. J Physiol 384:325–345
Bodega F, Sironi C, Armilli M, Porta C, Agostoni E (2009) Na+–glucose cotransporter is expressed by alveolar epithelial type I cells. Acta Physiol 197(Sup. 672):P19
Borok Z, Liebler JM, Lubman RL, Foster MJ, Zhou B, Li X, Zabski SM, Kim KJ, Crandall ED (2002) Na transport proteins are expressed by rat alveolar epithelial type I cells. Am J Physiol Lung Cell Mol Physiol 282:L599–L608
Boyd CAR (1990) Cellular basis of active d-glucose transport in mouse and rabbit lung. J Physiol 422:44P
de Prost N, Saumon G (2007) Glucose transport in the lung and its role in liquid movement. Respir Physiol Neurobiol 159:331–337
Dobbs LG, Johnson MD (2007) Alveolar epithelial transport in the adult lung. Respir Physiol Neurobiol 159:283–300
Dobbs LG, Williams MC, Brandt AE (1985) Changes in biochemical characteristics and pattern of lectin binding of alveolar type II cells with time in culture. Biochim Biophys Acta 846:155–166
Forssmann WG, Siegrist G, Orci L, Girardier L, Pictet R, Rouiller C (1967) Fixation par perfusion pour la microscopie électronique essai de généralisation. J Microscopie 6:279–304
Haies DM, Gil J, Weibel E (1981) Morphometric study of rat lung cells. Am Rev Respir Dis 123:533–541
Helms MN, Self J, Bao HF, Job LC, Jain L, Eaton D (2006) Dopamine activates-sensitive sodium channels in alveolar type I cells in lung slice preparations. Am J Physiol Lung Cell Mol Physiol 291:L610–L618
Johnson MD, Widdicombe JH, Allen L, Barbry P, Dobbs LG (2002) Alveolar epithelial type I cells contain transport proteins and transport sodium, supporting an active role for type I cells in regulation of lung liquid homeostasis. Proc Natl Acad Sci USA 99:1966–1971
Kemp PJ, Boyd CA (1992) Pathways for glucose transport in type II pneumocytes freshly isolated from adult guinea pig lung. Am J Physiol 263:L612–L616
Khoursandi S, Scharlau D, Herter P, Kuhnen C, Martin D, Kinne RK, Kipp H (2004) Different modes of sodium–d-glucose cotransporter-mediated d-glucose uptake regulation in Caco-2 cells. Am J Physiol Cell Physiol 287:1041–1047
Kipp H, Khoursandi S, Scharlau D, Kinne RK (2003) More than apical: distribution of SGLT1 in Caco-2 cells. Am J Physiol Cell Physiol 285:737–749
Mamchaoui K, Makhloufi Y, Saumon G (2002) Glucose transporter gene expression in freshly isolated and cultured rat pneumocytes. Acta Physiol Scand 175:19–24
Mason RJ, Dobbs LG (1980) Synthesis of phosphatidylcholine and phosphatidylglycerol by alveolar type II cells in primary culture. J Biol Chem 255:7372627
Saper CB (2009) A guide to the perplexed on the specificity of antibodies. J Histochem Cytochem 57:1–5
Saumon G, Martet G, Loiseau P (1996) Glucose transport and equilibrium across alveolar-airway barrier of rat. Am J Physiol 270:L183–L190
Schneeberger EE, McCarthy KM (1986) Cytochemical localization of Na-K-ATPase in rat type II pneumocytes. J Appl Physiol 60:1584–1589
Sironi C, Bodega F, Porta C, Zocchi L, Agostoni E (2007) Expression of Na+–glucose cotransporter (SGLT1) in visceral and parietal mesothelium of rabbit pleura. Respir Physiol Neurobiol 159:68–75
Sironi C, Bodega F, Porta C, Monaco A, Zocchi L, Agostoni E (2008) Na+–glucose cotransporter is also expressed in mesothelium of species with thick visceral pleura. Respir Physiol Neurobiol 161:261–266
Stone KC, Mercer RR, Gehr P, Stockstill B, Crapo J (1992) Allometric relationships of cell numbers and size in the mammalian lung. Am J Respir Cell Mol Biol 6:235–243
Strang LB (1991) Fetal lung liquid: secretion and reabsorption. Physiol Rev 71:991–1016
Taatjes DJ, Barcom LA, Leslie KO, Low RB (1990) Lectin binding pattern to terminal sugar of rat lung alveolar epithelial cells. J Histochem Citochem 38:233–244
Weibel ER (1973) Morphological basis of alveolar-capillary gas exchange. Physiol Rev 53:419–495
Weibel ER (1984) The pathway for oxygen. Harvard University Press, Cambridge, pp 314–315
Weibel ER (1985) Lung cell biology. In: Fishman AP, Fisher AB (eds) Handbook of physiology. The respiratory system circulation and nonrespiratory functions, section 3, vol 1. American Physiological Society, Bethesda, pp 47–91
Zhao FQ (2005) Cloning and expression of bovine sodium–glucose cotransporters. J Dairy Sci 88:182–194
Acknowledgments
We thank Dr. F. Acocella (Dipartimento di Scienze Cliniche Veterinarie, Università degli Studi di Milano) for providing the facilities of the Sezione di Clinica Chirurgica Veterinaria for lambs experiments, and Dr. A. Amadeo (Dipartimento di Scienze Biomolecolari e Biotecnologie, Università degli Studi di Milano) for providing the facilities of the laboratory of histology. We thank Dr. A. Monaco for his skilful assistance in lamb experiments, S. Bianchi for cryostat sections. Moreover, we are grateful to Dr. U. Fascio (Centro Interdipartimentale di Microscopia Avanzata, Università degli Studi di Milano) for confocal images acquisition. Finally, we thank R. Galli and P. Brioschi for their skilful technical assistance.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bodega, F., Sironi, C., Armilli, M. et al. Evidence for Na+–glucose cotransporter in type I alveolar epithelium. Histochem Cell Biol 134, 129–136 (2010). https://doi.org/10.1007/s00418-010-0725-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-010-0725-7