Abstract
Cation channels are of fundamental importance in regulating the function of airway smooth cells especially bronchoconstriction in response to spasmogens, and are therefore key players in the pathogenesis of asthma. To date, the identity of these cation channels remains a mystery. However, the recently emerged transient receptor potential (TRP) cation channel family has provided several promising channel candidates. The identification of the key TRP channels involved in regulating airway smooth muscle contractility, and therefore airway tone, could provide new and exciting prospects for the development of novel therapies for the treatment of airway diseases such as asthma.
Similar content being viewed by others
Introduction
Variable airflow obstruction caused by contraction of airways smooth muscle is a hallmark feature of asthma. In addition airway smooth muscle cell hyperplasia and hypertrophy are suggested to contribute to the development of the more persistent airway obstruction observed in chronic severe asthma (Elias et al. 1999). Ca2+ plays a central role in the pathophysiology of asthma, contributing to most mechanisms including excitation–contraction coupling of airway smooth muscle (ASM). In airway smooth muscle myocytes, increases in the cytosolic free calcium concentration ([Ca2+]i) act as a key determinant of force generation and cell proliferation (Rodger 1989; Panettieri 1998). A major mechanism for increasing the concentration of Ca2+ in the myoplasm is the influx of extracellular Ca2+ across the cell plasma membrane. The best characterised pathway of Ca2+ entry into airway smooth muscle cells is through dihydropyridine-sensitive L-type voltage-operated Ca2+ channels (VOCCs) whose presence has been shown at both whole cell and single channel levels (Kotlikoff 1988; Worley and Kotlikoff 1990). However, the ability of dihydropyridines and other VOCC blockers such as verapamil and diltiazem to attenuate contractions elicited by different spasmogens in guinea pig, rat and human airways has been variable. Thus while these compounds completely inhibit KCl-induced contractions they fail to inhibit or only partially inhibit contractile responses elicited by agonists such as histamine, endothelin 1, acetylcholine and leukotriene D4 (Gorenne et al. 1998; Bourdillat et al. 1987; Cuthbert et al. 1994; Oonuma et al. 2000). A particularly pertinent observation in the Gorenne et al. study, bearing in mind that the majority of asthmatics have allergic asthma, was that allergen-induced bronchoconstriction was insensitive to the L-VOCC blocker nifedipine.
Clinical studies on the effects of acute treatment of L-VOCC blockers have also produced similar results (Barnes 1985). These data suggest that L-VOCC blockers gave less protection against methacholine-induced bronchoconstriction than against histamine (Barnes 1985). The protective effect of nifedipine against histamine-induced bronchoconstriction administered systemically was thought to be modest because of the low doses used in order to minimise vascular side effects; however, the effect of delivering higher concentrations of nifedipine locally to the lung by nebulisation was no better, suggesting that the maximal effect of L-VOCC blockers on airway smooth muscle contraction was minimal (Barnes 1985). In general, L-VOCC-blocking drugs have proved disappointing in clinical trials for asthma (Lofdahl and Barnes 1986; Ferrari et al. 1989).
One possible explanation for the incomplete inhibition of agonist-induced airway smooth muscle contraction by L-VOCC blockers is the existence of additional Ca2+ entry pathways distinct from VOCCs, namely receptor-operated Ca2+ channels (ROCCs) and store-operated Ca2+ channels (SOCCs). These dihydropyridine-insensitive pathways have been described in human cultured tracheal smooth muscle cells (Murray and Kotlikoff 1991; Murray et al. 1993) and more recently have been demonstrated to be involved in spasmogen-induced Ca2+ influx and contraction in airway smooth muscle of large (2–5 mm diameters) and small (300–500 μm diameter) human bronchioles (Gorenne et al. 1998; Snetkov et al. 2001). In rat bronchioles Ca2+ entry triggered by depletion of the sarcoplasmic reticulum Ca2+ stores (also known as capacitative or store-operated Ca2+ entry) has also been implicated in mediating contraction and bronchial smooth muscle cell proliferation (Sweeney et al. 2002).
To date the identity of the ion channels mediating both receptor- and store-operated Ca2+ entry in airway smooth muscle have remained elusive. However, the recent emergence of the transient receptor potential (TRP) family of cation channel proteins has given fresh impetus to the identification of the molecular entities underpinning receptor-mediated Ca2+ influx in many cells types including airway smooth muscle cells.
Characteristics of Ca2+ entry pathways in airway smooth muscle
Studies of the properties of ROCCs and SOCCs in airway smooth muscle cells suggest that more than a single channel type is involved (Table 1) and in general they are easily differentiated from L-VOCCs. Unlike L-VOCCs, ROCCs and SOCCS are not activated by membrane depolarisation and are insensitive to L-VOCC blockers (Murray and Kotlikoff 1991). The mechanism by which ROCCs and SOCCs are activated as a result of G-protein-coupled receptor stimulation by receptor agonists such as methacholine or histamine in airway smooth muscle is not well understood. However, there is evidence that the products of phospholipase C activation, e.g. IP3 and diacylglycerol (DAG), are strongly implicated (see Barritt 1999 for a review on the subject). Differences in pharmacological sensitivities support the premise that airway smooth muscle expresses multiple ROCCs and/or SOCCs. For instance receptor-operated Ca2+ entry through some channels is only blocked by high concentrations (>100 μM) of lanthanide ions (Murray and Kotlikoff 1991) whilst other ROC pathways are almost completely inhibited by 1 μM La3+ (Ay et al. 2004).
In general, the evaluation of airway ROCCs lags behind vascular smooth muscle research, nevertheless, progress has been made in demonstrating their presence and possible functional roles in human airway smooth muscle cells and tissue. For example, the functional studies of Gorenne et al. (1998) on large human airways (2–5 mm) clearly demonstrate that the contractions elicited by respiratory-disease relevant spasmogens (LTD4, histamine, acetylcholine, anti-IgE) were largely dependent on SK&F 96365-sensitive ROCC-mediated Ca2+ influx. Furthermore, contractile and electrophysiological studies in small human bronchioles (<2 mm; Snetkov et al. 2001) demonstrate that ROCCs are also present in the lower respiratory tract and involved in contraction.
However, it is clear that more pharmacological and electrophysiological characterisation of the properties of these channels in airway smooth muscle is still required to determine how many different types of ROCCs are present. Questions regarding the distribution and function of these channels in the airways both across species and also in different regions of the bronchial tree are also absent.
TRPs as molecular candidates for ROCCs and SOCCs in airway smooth muscle
In light of the first publication providing compelling evidence for the functional role of a TRP channel as a ROCC in vascular smooth muscle function (Inoue et al. 2001), TRP channels have been obvious candidates in the most recent quests for the molecular identity of the ROCCs and SOCCs in airway smooth muscle. This has been fuelled by electrophysiological studies of airway smooth muscle, which have identified several non-selective cation channels, including one with conductance of ∼25 pS (Snetkov et al. 2001), a characteristic of several TRP channels (Clapham et al. 2003).
TRPC channels
The TRPC channels have attracted particular attention because these were the first mammalian TRP channels proposed as molecular candidates for ROCC and SOCC channels (Wes et al. 1995; Birnbaumer et al. 1996). In airway smooth muscle, TRPC1 is expressed in porcine, guinea pig, rat and human airway smooth muscle (Ay et al. 2004; Ong et al. 2003; Sweeney et al. 2002; Corteling et al. 2004). Interestingly, TRPC1 transcript expression appeared to be increased in proliferating rat bronchial myocytes suggesting that TRPC1 may play a role in airway smooth muscle proliferation and airway remodelling (Sweeney et al. 2002). TRPC3 and TRPC4 protein were also detected in porcine airway smooth muscle cells with TRPC3 as the predominant protein (Ay et al. 2004). Also of note is the controversy surrounding the ability of TRPC1 to form functional homomeric channels. An early study (Sinkins et al. 1998) suggested that TRPC1 could form homomeric channels; however, in other studies TRPC1 has failed to form functional channels unless co-expressed with TRPC4 or TRPC5, suggesting it can only express as a heteromultimeric complex (Strubing et al. 2001; Beech et al. 2003). In airway smooth muscle, mRNA expression of TRPCs 4 and 5 appears to differ between species (Table 1), e.g. the presence of TRPC5 mRNA in guinea-pig (Ong et al. 2003) but not in human airway smooth muscle (Corteling et al. 2004). These data suggest that the composition of TRPC proteins in airway smooth muscle differs between species. As TRPC1 can only form heteromeric complexes with either TRPC4 or TRPC5 (Hofmann et al. 2002; Goel et al. 2002), these observations suggest differential TRP isoform expression will determine which homomeric and heteromeric TRPC1/C4/C5 channels are possible in the native smooth muscle in each species and will determine the biophysical, regulatory and pharmacological properties of the functional channels.
The study of Corteling et al. (2004) demonstrated that TRPC6 mRNA was expressed in isolated human airway myocytes in addition to TRPC1, 3 and 4. Further analysis of TRPC6 expression confirmed that TRPC6 protein was present both in isolated human myocytes and in airway smooth muscle in human lung sections. Indirect evidence supporting a role for TRPC6 in airway smooth muscle function include data showing that, 20-hydroxyeicosatetraenoic acid (20-HETE) and 1-oleoyl-2-acetyl-sn-glycerol (OAG), which directly activate heterologously expressed TRPC6 channels (Hofmann et al. 1999; Basora et al. 2003), both activate non-selective cation currents in guinea-pig airway smooth muscle cells (Cloutier et al. 2003). Further studies also demonstrated that 20-HETE stimulated Ca2+ influx -dependent contraction of isolated guinea pig airway smooth muscle (Cloutier et al. 2003).
In summary, it appears that airway smooth muscle cells express a complement of several TRPC channels, yet their respective functional importance remains to be determined. However, the knowledge that TRPC proteins can form heteromultimers (Hofmann et al. 2002; Goel et al. 2002) raises the possibility of several different functional TRPC channels in airway smooth muscle that are dependent upon differential expression of the individual proteins, which could in theory be dynamically regulated by factors that change TRP gene expression, e.g. during disease.
TRPV channels
The TRPV subfamily, containing six members (TRPV1–6) are cation selective channels, which are Ca2+ permeable and expressed widely in both excitable and non-excitable cells including the heart, lung, kidney, nerves and endothelial cells (Vennekens et al. 2002; O’Neil and Brown 2003). In contrast to the TRPC family, which has been the focus much attention in the smooth muscle field, the TRPV channels are positively promiscuous in the stimuli to which they respond. Whereas the TRPCs are predominantly activated as a result of Gq-coupled receptor occupation or store depletion, the members of the TRPV family can be activated by diverse stimuli including protons, irritants, lipids, mechanical stimuli, noxious heat and changes in cell volume (Gunthorpe et al. 2002). It is perhaps the latter property, responsiveness to changes in cell volume, that led Jia et al. (2004) to look at the expression and function of TRPV channels in airway smooth muscle (Table 1).
For many years it has been known that decreasing the osmolarity of the airway surface liquid by inhalation of hypotonic aerosols is a potent stimulus for airway narrowing in asthmatics (Allegra and Bianco 1980; Anderson et al. 1983), aggravating the bronchial hyper-responsiveness that is a characteristic hallmark of the disease. The mechanism by which hypotonicity elicits this response is not clear, however, the effect does persist when airways are isolated (Finney et al. 1987). Two members of the TRPV family, TRPV2 and TRPV4, when expressed in heterologous systems have been shown to activate in response to hypotonic shock (Muraki et al. 2003; Liedtke et al. 2000; Strotmann et al. 2000). Jia et al. (2004) report that the message for both TRPV2 and V4 are present in primary cultured human bronchial smooth muscle cells (HBSMC) and that hypotonicity-induced constriction of human bronchi is dependent upon the presence of extracellular Ca2+, a finding that can be recapitulated in guinea-pig airways. Their study shows hypotonicity-induced increases in Ca2+ in cultured HBSMC, an effect that can be mimicked by the phorbol derivative 4alpha-phorbol 12,13-didecanoate (4α-PDD), reported to be a TRPV4 activator (Watanabe et al. 2002). The action of 4α-PDD was antagonised by pre-treatment with ruthenium red, a putative TRPV channel blocker. However, the effectiveness of ruthenium red versus a hypotonic challenge was not assessed, and due to its reported inhibitory effect upon myosin light chain phosphatase (Yamada et al. 2000), ruthenium red is not a suitable pharmacological tool for contractile studies involving smooth muscle.
Although the data provided by Jia et al. (2004) provide strong evidence for the role of TRPV4 in hypotonicity-induced bronchial contraction, TRPV2, whose message was also detected in HBSMCs, cannot be completely excluded. Discriminating pharmacological tools for both TRPVs are not yet available; however, trpv4-/- mice are viable and exhibit a phenotype consistent with disruption of a CNS-based osmosensor (Liedtke and Friedman 2003). To date their lung function and smooth muscle responses remain unexplored and these data and their insight into the peripheral role of TRPV4 is eagerly awaited.
Although asthmatics bronchoconstrict in response to inhaled distilled water or hypotonic saline, normal subjects do not (Schoeffel et al. 1981). Normally the respiratory epithelium, characterised by tight junctions, is proposed to insulate the underlying smooth muscle cells from changes in the airway surface liquid composition. However, in asthma there is widespread airway remodelling, which includes epithelial denudation (Laitinen et al. 1985). This pathological process may result in direct exposure of the smooth muscle to the airway surface liquid and hence lead to activation of muscle resident osmosensors such as TRPV4. It is, however, also important to consider the wider role of TRPV4 in the lung. TRPV4 has been reported to be expressed by a human bronchial epithelial cell line (Fernandez-Fernandez et al. 2002) and proposed to play a fundamental role in volume regulation in these cells. Is TRPV4 expressed by sensory nerves in the airways, which may also be exposed to the airway surface liquid by epithelial denudation? Are the levels of TRPV4 expressed by airway smooth muscle affected by respiratory disease? The study by Jia et al. (2004) certainly suggests that TRPV4 has a role to play in airway smooth muscle contraction and raises the intriguing possibility of a novel target for the treatment of asthma. However, a definitive link between molecular identity and ascribed channel function is not yet attained.
For TRPV2, also expressed by human airways, a role remains undefined. The translocation of TRPV2 from intracellular pools to the cell membrane in response to stimulation by growth factors such as insulin-like growth factor I (IGF-I) is intriguing (Kanzaki et al. 1999), but not unique within the TRP family as TRPC5 has recently been reported to undergo ‘rapid vesicular insertion’ in response to epidermal growth factor (EGF; Bezzerides et al. 2004). In human airways, IGF-I is reported to induce both contraction and proliferation of smooth muscle (Gosens et al. 2004; Cohen et al. 1995), the induced contraction being slow to develop, yet sustained. It is tempting to speculate that TRPV2 may contribute to the IGF-I mediated effects and the role of TRPV2 in the airways warrants further investigation.
TRPM channels
To our knowledge, even less is known about the distribution and potential role of TRPM channels in airway smooth muscle than either the TRPCs or TRPVs. Although TRPM2 is expressed in lung (Nagamine et al. 1998; Hara et al. 2002), there are no published data on TRPM2 expression or function in airway smooth muscle. TRPM channels, which may be of functional significance include TRPM3, which like TRPV2 and TRPV4, is a Ca2+-permeable cation channel regulated by changes in cellular osmolarity (Grimm et al. 2003; Liedtke and Simon 2004; Fleig and Penner 2004). In addition, both TRPM4 and TRPM5 have been shown to regulate membrane depolarisation (Launay et al. 2002; Hofmann et al. 2003). If expressed in airway smooth muscle cells, they could modulate Ca2+ influx via both voltage-dependent and voltage-independent Ca2+-permeable channels. Clearly with such a gap in our knowledge of TRPM channels in airway smooth muscle, there is ample opportunity to explore their functional significance in this cell type.
Conserved functional roles for TRP channels in smooth muscle?
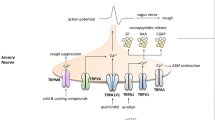
Historically, research in the area of vascular smooth muscle has outpaced that of airway and other smooth muscle beds, possibly as a result of cardiovascular disease being the major cause of mortality worldwide. The field of store and receptor-operated cation channels is no exception and the physiological role of the TRP channels, the putative molecular candidates, is more advanced in blood vessels than airways. A recent review by Beech et al. (2004) provides an excellent summary of the field of TRP channels in smooth muscle. Even though airway smooth muscle has been shown to express mRNA for as many different TRP channels as the vasculature, the number of functional studies characterising non-selective cation channels in the vasculature far exceeds those performed in the airways (Beech et al. 2004). As respiratory disease is increasing in prevalence worldwide, it is perhaps time that this imbalance were re-addressed. However, the vascular field may provide valuable functional insights and useful approaches for discerning the roles of the TRP superfamily in airways. The current knowledge of signalling pathways involving TRP channels in airway smooth muscle cells are summarised in Fig. 1.
Calcium influx pathways in airway smooth muscle cells. 4α-PDD, 4α-phorbol 12,13-didecanoate; AA, arachidonic acid; CICR, calcium-induced calcium release; DAG, diacylglycerol; EET, eicosatrienoic acid; Em, membrane potential; IP3, inositol-1,4,5-triphosphate; IP3R, inositol-1,4,5-triphosphate receptor; PLA2, phospholipase A2; PLC, phospholipase C; RyR, ryanodine receptor; SOCC, store-operated calcium channel; TRPC, transient receptor potential canonical; TRPV, transient receptor potential vanilloid receptor-related protein; VOCC, voltage-operated calcium channel
In the pulmonary vasculature the work of Jason Yuan and colleagues has clearly demonstrated roles for TRP channels not only in the genesis of tone but in pulmonary artery smooth muscle cell (PASMC) proliferation, which may have significant clinical relevance in pulmonary hypertension (Landsberg and Yuan 2004). Yu et al. (2003) show that targeted reduction of TRPC6 expression by antisense oligonucleotides inhibited platelet derived growth factor (PDGF)-mediated proliferation of PASMC. The levels of both TRPC3, normally expressed at extremely low levels, and TRPC6 transcripts and proteins are significantly higher in the PASMC of idiopathic pulmonary arterial hypertension (IPAH) patients in comparison to both normals and patients diagnosed with secondary pulmonary arterial hypertension (SPAH; Yu et al. 2004). Mitogens, including PDGF, are proposed to be key contributors to the remodelling process associated with severe asthma and have been clearly shown to induce airway smooth muscle cell hyperplasia. However, there is a paucity of information regarding the roles or changes in expression profile of ion channels in proliferating ASM, and it will be interesting to see if the proposed role of certain TRPC channels in PASMC translates to airway smooth muscle.
Control of smooth muscle tone offers important therapeutic avenues in both cardiovascular and respiratory disease. Inoue et al. (2001) reported that the dominant TRPC channel expressed in rabbit portal vein was TRPC6 and that the properties of this channel matched those of the native α1-adrenoceptor-activated Ca2+ permeable non-selective cation channel (α1-NSCC). Employing specific anti-sense oligonucleotides Inoue et al. showed that reduction of TRPC6 immunoreactivity in cultured rabbit portal vein myocytes was accompanied by a reduction in functional readouts, both the phenylephrine stimulated cation current and Ca2+ influx were significantly reduced. This led Inoue to conclude that TRPC6 has a fundamental role to play in the control of systemic blood pressure via sympathetic nerves. Unfortunately the authors did not present any contractile data to fully support their conclusion. An analogous approach was adopted by Brayden and colleagues (Welsh et al. 2002) who used antisense to demonstrate a functional role for TRPC6 in generation of myogenic tone in rat cerebral resistance arteries. As our own studies have shown TRPC6 is expressed in human airway smooth muscle (Corteling et al. 2004) it is tempting to speculate that it fulfils a similar role and may offer therapeutic potential in reducing airway constriction. However, the eagerly awaited data recently reported by Thomas Gudermann’s group who have generated a trpc6-/- mouse has highlighted the complexity and challenges of TRP channel research (Dietrich et al. 2005 in press). Unexpectedly the trpc6-/- mouse has both elevated airway resistance and mean arterial blood pressure. Analysis of vascular and airway vessels demonstrate an enhanced contractility and sensitivity to constrictor stimuli, a phenotype expected for over-expression rather than ablation of a TRPC channel. Although still under investigation, this has led the authors to conclude that removal of the TRPC6 gene has resulted in higher basal activities or upregulation of other members of the TRPC family as a consequence.
Future challenges and opportunities
As smooth muscles, including airway, express a multitude of TRP channels, the complexity in assessing which are the important players in generating tone, and which, if any, are redundant innocent bystanders in the process, is a real and present challenge. Heteromultimer formation, as demonstrated for members of the TRPC family, maybe a double-edged sword, complicating ascribing physiological function whilst concomitantly offering an enhanced opportunity for tissue-specific combinations which could be therapeutically exploited. The relative ionic permeability of the TRP channels themselves adds to the intrigue, further complicating interpretation of physiological role. For channels with high Ca2+-selectivity ratios this is easier to reconcile, the most obvious conclusion being a direct role in extracellular Ca2+ entry. However, for TRP channels, which are much less discriminating in their permeability profile (i.e. non-selective cation channels), such as certain members of the TRPM family, the situation is not so clear. Activation of these channels will, in addition to allowing Ca2+ influx, elicit a substantial depolarisation, which in airway smooth muscle may serve to activate L-VOCCs and simultaneously limit Ca2+ entry by attenuating the electrical driving force for Ca2+ entry.
Clearly, much remains to be done to delineate the functional roles of TRPs in airway smooth muscle. However, identification of the TRP channels involved in regulating airway smooth muscle contractility offers the exciting prospect of new and novel therapies for the treatment of airway diseases such as asthma.
References
Allegra L, Bianco S (1980) Non-specific broncho-reactivity obtained with an ultrasonic aerosol of distilled water. Eur J Respir Dis Suppl 106:41–49
Anderson SD, Schoeffel RE, Finney M (1983) Evaluation of ultrasonically nebulised solutions for provocation testing in patients with asthma. Thorax 38:284–291
Ay B, Prakash YS, Pabelick CM, Sieck GC (2004) Store-operated Ca2+ entry in porcine airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 286:L909–L917
Barnes PJ (1985) Clinical studies with calcium antagonists in asthma. Br J Clin Pharmacol 20 [Suppl 2]:289S–298S
Barritt GJ (1999) Receptor-activated Ca2+ inflow in animal cells: a variety of pathways tailored to meet different intracellular Ca2+ signalling requirements. Biochem J 337(Pt 2):153–169
Basora N, Boulay G, Bilodeau L, Rousseau E, Payet MD (2003) 20-Hydroxyeicosatetraenoic acid (20-HETE) activates mouse TRPC6 channels expressed in HEK293 cells. J Biol Chem 278:31709–31716
Beech DJ, Xu SZ, McHugh D, Flemming R (2003) TRPC1 store-operated cationic channel subunit. Cell Calcium 33:433–440
Beech DJ, Muraki K, Flemming R (2004) Non-selective cationic channels of smooth muscle and the mammalian homologues of Drosophila TRP. J Physiol 559:685–706
Bezzerides VJ, Ramsey IS, Kotecha S, Greka A, Clapham DE (2004) Rapid vesicular translocation and insertion of TRP channels. Nat Cell Biol 6:709–720
Birnbaumer L, Zhu X, Jiang M, Boulay G, Peyton M, Vannier B, Brown D, Platano D, Sadeghi H, Stefani E, Birnbaumer M (1996) On the molecular basis and regulation of cellular capacitative calcium entry: roles for Trp proteins. Proc Natl Acad Sci USA 93:15195–15202
Bourdillat B, Haye-Legrand I, Labat C, Raffestin B, Norel X, Benveniste J, Brink C (1987) Effects of various pharmacological agents on isolated human bronchial and pulmonary arterial and venous muscle preparations contracted by leukotriene D4. Fundam Clin Pharmacol 1:433–444
Clapham DE, Montell C, Schultz G, Julius D (2003) International Union of Pharmacology. XLIII. Compendium of voltage-gated ion channels: transient receptor potential channels. Pharmacol Rev 55:591–596
Cloutier M, Campbell S, Basora N, Proteau S, Payet MD, Rousseau E (2003) 20-HETE inotropic effects involve the activation of a nonselective cationic current in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 285:L560–L568
Cohen P, Noveral JP, Bhala A, Nunn SE, Herrick DJ, Grunstein MM (1995) Leukotriene D4 facilitates airway smooth muscle cell proliferation via modulation of the IGF axis. Am J Physiol 269:L151–L157
Corteling RL, Li S, Giddings J, Westwick J, Poll C, Hall IP (2004) Expression of transient receptor potential C6 and related transient receptor potential family members in human airway smooth muscle and lung tissue. Am J Respir Cell Mol Biol 30:145–154
Cuthbert NJ, Gardiner PJ, Nash K, Poll CT (1994) Roles of Ca2+ influx and intracellular Ca2+ release in agonist-induced contractions in guinea pig trachea. Am J Physiol 266:L620–L627
Dietrich A, Mederos y Schnitzler M, Kalwa H, Storch U, Gudermann T (2005) Functional characterization and physiological relevance of the TRPC3/6/7 subfamily of cation channels. Naunyn-Schmiedebergs Arch Pharmacol (in press)
Elias JA, Zhu Z, Chupp G, Homer RJ (1999) Airway remodeling in asthma. J Clin Invest 104:1001–1006
Fernandez-Fernandez JM, Nobles M, Currid A, Vazquez E, Valverde MA (2002) Maxi K+ channel mediates regulatory volume decrease response in a human bronchial epithelial cell line. Am J Physiol Cell Physiol 283:C1705–C1714
Ferrari M, Olivieri M, De Gasperi M, Lechi A (1989) Differential effects of nifedipine and diltiazem on methacholine-induced bronchospasm in allergic asthma. Ann Allergy 63:196–200
Finney MJ, Anderson SD, Black JL (1987) The effect of non-isotonic solutions on human isolated airway smooth muscle. Respir Physiol 69:277–286
Fleig A, Penner R (2004) The TRPM ion channel subfamily: molecular, biophysical and functional features. Trends Pharmacol Sci 25:633–639
Goel M, Sinkins WG, Schilling WP (2002) Selective association of TRPC channel subunits in rat brain synaptosomes. J Biol Chem 277:48303–48310
Gorenne I, Labat C, Gascard JP, Norel X, Nashashibi N, Brink C (1998) Leukotriene D4 contractions in human airways are blocked by SK&F 96365, an inhibitor of receptor-mediated calcium entry. J Pharmacol Exp Ther 284:549–552
Gosens R, Schaafsma D, Grootte Bromhaar MM, Vrugt B, Zaagsma J, Meurs H, Nelemans SA (2004) Growth factor-induced contraction of human bronchial smooth muscle is rho-kinase-dependent. Eur J Pharmacol 494:73–76
Grimm C, Kraft R, Sauerbruch S, Schultz G, Harteneck C (2003) Molecular and functional characterization of the melastatin-related cation channel TRPM3. J Biol Chem 278:21493–21501
Gunthorpe MJ, Benham CD, Randall A, Davis JB (2002) The diversity in the vanilloid (TRPV) receptor family of ion channels. Trends Pharmacol Sci 23:183–191
Hara Y, Wakamori M, Ishii M, Maeno E, Nishida M, Yoshida T, Yamada H, Shimizu S, Mori E, Kudoh J, Shimizu N, Kurose H, Okada Y, Imoto K, Mori Y (2002) LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell 9:163–173
Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G (1999) Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 397:259–263
Hofmann T, Schaefer M, Schultz G, Gudermann T (2002) Subunit composition of mammalian transient receptor potential channels in living cells. Proc Natl Acad Sci USA 99:7461–7466
Hofmann T, Chubanov V, Gudermann T, Montell C (2003) TRPM5 is a voltage-modulated and Ca(2+)-activated monovalent selective cation channel. Curr Biol 13:1153–1158
Inoue R, Okada T, Onoue H, Hara Y, Shimizu S, Naitoh S, Ito Y, Mori Y (2001) The transient receptor potential protein homologue TRP6 is the essential component of vascular alpha(1)-adrenoceptor-activated Ca2+-permeable cation channel. Circ Res 88:325–332
Ito S, Kume H, Yamaki K, Katoh H, Honjo H, Kodama I, Hayashi H (2002) Regulation of capacitative and noncapacitative receptor-operated Ca2+ entry by rho-kinase in tracheal smooth muscle. Am J Respir Cell Mol Biol 26:491–498
Jia Y, Wang X, Varty L, Rizzo CA, Yang R, Correll CC, Phelps PT, Egan RW, Hey JA (2004) Functional TRPV4 channels are expressed in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 287:L272–L278
Kanzaki M, Zhang YQ, Mashima H, Li L, Shibata H, Kojima I (1999) Translocation of a calcium-permeable cation channel induced by insulin-like growth factor-I. Nat Cell Biol 1:165–170
Kotlikoff MI (1988) Calcium currents in isolated canine airway smooth muscle cells. Am J Physiol 254:C793–C801
Laitinen LA, Heino M, Laitinen A, Kava T, Haahtela T (1985) Damage of the airway epithelium and bronchial reactivity in patients with asthma. Am Rev Respir Dis 131:599–606
Landsberg JW, Yuan JX (2004) Calcium and TRP channels in pulmonary vascular smooth muscle cell proliferation. News Physiol Sci 19:44–50
Launay P, Fleig A, Perraud AL, Scharenberg AM, Penner R, Kinet JP (2002) TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell 109:397–407
Liedtke W, Friedman JM (2003) Abnormal osmotic regulation in trpv4-/- mice. Proc Natl Acad Sci USA 100:13698–13703
Liedtke W, Simon SA (2004) A possible role for TRPV4 receptors in asthma. Am J Physiol Lung Cell Mol Physiol 287:L269–L271
Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S (2000) Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 103:525–535
Lofdahl CG, Barnes PJ (1986) Calcium, calcium channel blockade and airways function. Acta Pharmacol Toxicol (Copenh) 58 [Suppl 2]:91–111
Muraki K, Iwata Y, Katanosaka Y, Ito T, Ohya S, Shigekawa M, Imaizumi Y (2003) TRPV2 is a component of osmotically sensitive cation channels in murine aortic myocytes. Circ Res 93:829–838
Murray RK, Kotlikoff MI (1991) Receptor-activated calcium influx in human airway smooth muscle cells. J Physiol 435:123–144
Murray RK, Fleischmann BK, Kotlikoff MI (1993) Receptor-activated Ca influx in human airway smooth muscle: use of Ca imaging and perforated patch-clamp techniques. Am J Physiol 264:C485–C490
Nagamine K, Kudoh J, Minoshima S, Kawasaki K, Asakawa S, Ito F, Shimizu N (1998) Molecular cloning of a novel putative Ca2+ channel protein (TRPC7) highly expressed in brain. Genomics 54:124–131
O’Neil RG, Brown RC (2003) The vanilloid receptor family of calcium-permeable channels: molecular integrators of microenvironmental stimuli. News Physiol Sci 18:226–231
Ong HL, Brereton HM, Harland ML, Barritt GJ (2003) Evidence for the expression of transient receptor potential proteins in guinea pig airway smooth muscle cells. Respirology 8:23–32
Oonuma H, Nakajima T, Nagata T, Iwasawa K, Wang Y, Hazama H, Morita Y, Yamamoto K, Nagai R, Omata M (2000) Endothelin-1 is a potent activator of non-selective cation currents in human bronchial smooth muscle cells. Am J Respir Cell Mol Biol 23:213–221
Panettieri RA Jr (1998) Cellular and molecular mechanisms regulating airway smooth muscle proliferation and cell adhesion molecule expression. Am J Respir Crit Care Med 158:S133–S140
Rodger I (1989) Drugs affecting calcium ions in airway smooth muscle. In: Barnes PJ (ed) New drugs for asthma. IBC, London, pp 455–456
Rousseau E, Cloutier M, Morin C, Proteau S (2005) Capsazepine, a vanilloid antagonist, abolishes tonic responses induced by 20-HETE on guinea pig airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 288:L460–L470
Schoeffel RE, Anderson SD, Altounyan RE (1981) Bronchial hyperreactivity in response to inhalation of ultrasonically nebulised solutions of distilled water and saline. Br Med J (Clin Res Ed) 283:1285–1287
Sinkins WG, Estacion M, Schilling WP (1998) Functional expression of TrpC1: a human homologue of the Drosophila Trp channel. Biochem J 331(Pt 1):331–339
Snetkov VA, Hapgood KJ, McVicker CG, Lee TH, Ward JP (2001) Mechanisms of leukotriene D4-induced constriction in human small bronchioles. Br J Pharmacol 133:243–252
Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD (2000) OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol 2:695–702
Strubing C, Krapivinsky G, Krapivinsky L, Clapham DE (2001) TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron 29:645–655
Sweeney M, McDaniel SS, Platoshyn O, Zhang S, Yu Y, Lapp BR, Zhao Y, Thistlethwaite PA, Yuan JX (2002) Role of capacitative Ca2+ entry in bronchial contraction and remodeling. J Appl Physiol 92:1594–1602
Vennekens R, Voets T, Bindels RJ, Droogmans G, Nilius B (2002) Current understanding of mammalian TRP homologues. Cell Calcium 31:253–264
Watanabe H, Davis JB, Smart D, Jerman JC, Smith GD, Hayes P, Vriens J, Cairns W, Wissenbach U, Prenen J, Flockerzi V, Droogmans G, Benham CD, Nilius B (2002) Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem 277:13569–13577
Welsh DG, Morielli AD, Nelson MT, Brayden JE (2002) Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ Res 90:248–250
Wes PD, Chevesich J, Jeromin A, Rosenberg C, Stetten G, Montell C (1995) TRPC1, a human homolog of a Drosophila store-operated channel. Proc Natl Acad Sci USA 92:9652–9656
Worley JF III, Kotlikoff MI (1990) Dihydropyridine-sensitive single calcium channels in airway smooth muscle cells. Am J Physiol 259:L468–L480
Yamada A, Sato O, Watanabe M, Walsh MP, Ogawa Y, Imaizumi Y (2000) Inhibition of smooth-muscle myosin-light-chain phosphatase by ruthenium red. Biochem J 349(Pt 3):797–804
Yu Y, Sweeney M, Zhang S, Platoshyn O, Landsberg J, Rothman A, Yuan JX (2003) PDGF stimulates pulmonary vascular smooth muscle cell proliferation by upregulating TRPC6 expression. Am J Physiol Cell Physiol 284:C316–C330
Yu Y, Fantozzi I, Remillard CV, Landsberg JW, Kunichika N, Platoshyn O, Tigno DD, Thistlethwaite PA, Rubin LJ, Yuan JX (2004) Enhanced expression of transient receptor potential channels in idiopathic pulmonary arterial hypertension. Proc Natl Acad Sci USA 101:13861–13866
Acknowledgements
The authors thank Dr Anne Dodge, Novartis Institutes for Biomedical Research Horsham, for her expert review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gosling, M., Poll, C. & Li, S. TRP channels in airway smooth muscle as therapeutic targets. Naunyn-Schmiedeberg's Arch Pharmacol 371, 277–284 (2005). https://doi.org/10.1007/s00210-005-1058-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-005-1058-2