Abstract
Purpose
Information about healthcare-associated pneumonia (HCAP) in critically ill patients is scarce.
Methods
This prospective study compared clinical presentation, outcomes, microbial etiology, and treatment of HCAP, community-acquired pneumonia (CAP), and immunocompromised patients (ICP) with severe pneumonia admitted to 34 Spanish ICUs.
Results
A total of 726 patients with pneumonia (449 CAP, 133 HCAP, and 144 ICP) were recruited during 1 year from April 2011. HCAP patients had more comorbidities and worse clinical status (Barthel score). HCAP and ICP patients needed mechanical ventilation and tracheotomy more frequently than CAP patients. Streptococcus pneumoniae was the most frequent pathogen in all three groups (CAP, 34.2 %; HCAP, 19.5 %; ICP, 23.4 %; p = 0.001). The overall incidence of Gram-negative pathogens, methicillin-resistant Staphylococcus aureus (MRSA), and Pseudomonas aeruginosa was low, but higher in HCAP and ICP patients than CAP. Empirical treatment was in line with CAP guidelines in 73.5 % of patients with CAP, in 45.5 % of those with HCAP, and in 40 % of those with ICP. The incidence of inappropriate empirical antibiotic therapy was 6.5 % in CAP, 14.4 % in HCAP, and 21.8 % in ICP (p < 0.001). Mortality was highest in ICP (38.6 %) and did not differ between CAP (18.4 %) and HCAP (21.2 %).
Conclusions
HCAP accounts for one-fifth of cases of severe pneumonia in patients admitted to Spanish ICUs. The empirical antibiotic therapy recommended for CAP would be appropriate for 90 % of patients with HCAP in our population, and consequently the decision to include coverage of multidrug-resistant pathogens for HCAP should be cautiously judged in order to prevent the overuse of antimicrobials.
Similar content being viewed by others
Introduction
The 2005 update of the American Thoracic Society (ATS)/Infectious Disease Society of America’s (IDSA) guidelines on nosocomial pneumonia [1] introduced the category healthcare-associated pneumonia (HCAP) for pneumonia occurring in outpatients at risk of infections with resistant pathogens through contact with the healthcare system. This category includes patients who were recently hospitalized, residence in a nursing home or extended-care facility, undergoing chronic dialysis, or recently received wound care or infusion therapy at home.
Apparently, epidemiology and causative pathogens of HCAP differ from those of community-acquired pneumonia (CAP); it seems that HCAP is more often caused by potentially drug-resistant pathogens commonly seen in hospital-acquired infections [2]. Thus, patients with HCAP would require broader antibiotic coverage than those with CAP to reduce the risk of initially inadequate antibiotic therapy and worsened outcomes.
However, several studies about HCAP reveal epidemiological variations that seem to follow a geographic distribution. Studies done in the USA and Asia report a high frequency of multidrug-resistant pathogens (methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa) [2–5]; by contrast most European (Spain, UK) studies report a high frequency of pathogens resembling those causing CAP (Streptococcus pneumoniae being the most frequent pathogen) [6–8], suggesting that the empiric antibiotic treatment prescribed for CAP is still adequate for most European HCAP.
In both the American and European studies, mortality was higher in patients with HCAP than in those with CAP; however, it is unclear whether mortality was higher because patients with HCAP received inadequate antimicrobial treatment or because they were older, and had more comorbidities and treatment restrictions. Furthermore, most information about HCAP in USA and Europe comes from patients who were not admitted to the intensive care unit (ICU) [2–8].
We aimed to describe the epidemiology, clinical features, and outcomes of HCAP in a homogeneous population of critically ill patients admitted to 34 Spanish ICUs with severe pneumonia.
Methods
Study design
This prospective, observational study was performed in 34 ICUs in urban teaching hospitals in Spain.
All consecutive patients older than 18 years admitted to the participating ICUs with severe pneumonia between 1 April 2011 and 31 March 2012 were eligible. Clinical, epidemiological, and laboratory data were prospectively recorded using a standardized worksheet and stored in a computer database. We excluded all episodes of pneumonia diagnosed more than 48 h after admission to the ICU. The ethics committees at the participating hospitals approved the study protocol, and patients or their relatives provided written informed consent.
Data collection
At least one predesignated physician with specific expertise in infectious diseases at each institution evaluated each patient to confirm the diagnosis of pneumonia on the basis of the patient’s history, body temperature, and findings of physical examination, laboratory analyses, chest X-ray, and microbiology.
We collected data on demographic characteristics, risk factors prior to hospitalization, blood cultures, susceptibility testing and appropriateness of empiric antibiotic treatment, systemic response, date of ICU admission, and date of ICU discharge or death. Underlying diseases and severity at admission were classified according to the APACHE II score [9] and SOFA score [10]. We also recorded information about pneumonia severity, such as PSI score [11], CURB-65 score [12], and major and minor ATS criteria of severe pneumonia [13]. Previous functional status was evaluated by Barthel score [14]. We also recorded pneumonia-related complications, such as pleural effusion, empyema, acute respiratory distress syndrome (ARDS), need for invasive or non-invasive mechanical ventilation and tracheotomy, acute renal injury (serum creatinine increase to 2.0-fold or GFR decrease greater than 50 % from baseline and/or urine output less than 0.5 ml/kg for at least 12 h), and disseminated intravascular coagulation (DIC) (platelet count less than 100 × 109/L, fibrinogen less than 1 g/L, and prothrombin time ratio greater than 1.6). We calculated the incidence of treatment restrictions (advanced directives and “do-not-resuscitate” orders) and crude mortality in the ICU.
Definitions
Pneumonia was diagnosed when both signs and symptoms of pulmonary infection together with infiltrate(s) on chest X-rays were present at ICU admission. Following the ATS/IDSA guidelines [1], we classified pneumonia as HCAP or CAP; we classified pneumonia in immunocompromised patients (ICP) as a separate entity.
Pneumonia in patients fulfilling any of the following criteria was classified as HCAP [1]:
-
1.
Hospitalization in an acute care hospital for 48 h or longer in the 90 days before the pneumonia
-
2.
Residence in a nursing home or extended-care facility
-
3.
Home infusion therapy (including antibiotics)
-
4.
Chronic dialysis within 30 days
-
5.
Home wound care
-
6.
Family member with multidrug-resistant pathogens.
Pneumonia in immunocompromised patients was classified as ICP defined by immunosuppressive treatment, chemotherapy, or corticosteroid therapy for at least 4 weeks before the diagnosis of pneumonia, had received an organ transplant, or were HIV-positive.
Pneumonia that did not fulfill the criteria for HCAP or ICP was classified as CAP.
In the HCAP group we recorded antibiotic therapy in the 6 months before admission to the ICU.
We assessed comorbidity by reviewing medical records for diabetes, chronic obstructive pulmonary disease, chronic renal failure, chronic liver disease, alcoholism, stroke, dementia, injected drug abuse, active cancer, transplantation, or HIV infection. Therapy for the pneumonia was considered appropriate when at least one effective drug was included in the empirical antibiotic treatment within the first 24 h of the diagnosis [15]. CAP guidelines recommended by ATS/IDSA were used to evaluate the empirical antibiotic treatment [13]. Antibiotic treatment was prescribed at the discretion of the attending physician.
Microbiology
The microbiological diagnosis of pneumonia required a positive culture of blood, pleural fluid, or sputum, or in intubated patients, of bronchoscopic specimens from the lower airways. In these cases, cultures were considered positive when the colony count was at least 106 cfu/mL from cultures of tracheobronchial aspirate samples, at least 103 cfu/mL from cultures of protected specimen brush (PSB) samples, or at least 104 cfu/mL from cultures of bronchoalveolar lavage (BAL) samples. We also considered a positive urinary antigen test for either S. pneumoniae or Legionella as evidence of a bacterial infection. Pleural effusion cultures were obtained in patients with pleural effusion. Bacterial identification and susceptibility testing were performed by standard methods in accordance with the Clinical and Laboratory Standards Institute’s (CLSI) performance standards [16]. MRSA, P. aeruginosa, Stenotrophomonas maltophilia, Acinetobacter baumannii, Klebsiella sp., and Serratia marcescens were considered potentially resistant microorganisms (PRMO). In bronchoalveolar lavage specimens, Pneumocystis jiroveci was identified by direct immunofluorescence (IFA) and cytomegalovirus by polymerase chain reaction (PCR). Nasal and throat swabs were analyzed for respiratory virus by PCR at the discretion of the attending physicians. Serologic tests were used to detect atypical organisms, and a fourfold increase in antibody levels was considered to establish a diagnosis.
Statistical analysis
Discrete variables are expressed as counts (percentage) and continuous variables as mean ± standard deviation. Differences in demographic and clinical characteristics between groups were assessed using the Chi-squared test and Fisher’s exact test for categorical variables and Student’s t test or the Mann–Whitney U test for continuous variables. Means were compared using ANOVA. The impact of independent variables on ICU mortality was assessed by multivariate logistic regression analysis. To avoid spurious associations, only variables significantly associated in the univariate analysis (p ≤ 0.05) or having a plausible relationship with the dependent variable were included in the multivariate analysis. Potential explanatory variables were checked for colinearity using the tolerance and variance inflation factor prior to inclusion in the regression models [17]. Results are presented as odds ratios (OR) and 95 % confidence intervals (CI). Data was analyzed using SPSS for Windows 15.0 (SPSS, Chicago, IL, US).
Results
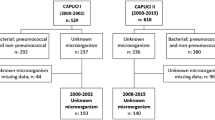
During the study period, 728 patients with pneumonia were admitted to the 34 participating ICUs, and two patients were excluded because of incomplete data (Fig. 1). In total, 726 patients were included, and pneumonia was classified as CAP in 449 (61.8 %), as HCAP in 133 (18.3 %), and as ICP in 144 (19.8 %).
Baseline demographics and clinical characteristics differed among the three subgroups of patients (Table 1). Patients with HCAP were older, had worse functional status (Barthel index), and had a higher frequency of COPD, congestive heart failure, and diabetes. Patients with ICP had a higher incidence of active cancer and chronic liver failure.
The reasons for classifying pneumonia as HCAP were hospitalization within the preceding 3 months (53.7 %), residence in a long-term-care facility (27.7 %), home infusion therapy or home wound care (12.0 %), and outpatient hemodialysis (6.5 %). No patients were classified as HCAP due to family colonized with resistant pathogens. A total of 51.8 % of HCAP patients had received antibiotic therapy in the 6 months before admission to the ICU.
Severity of pneumonia and complications
Patients with HCAP and ICP had higher initial APACHE II and SOFA scores than patients with CAP (Table 2). On the specific severity indices for pneumonia, CURB-65 scores were highest in patients with HCAP, and PSI scores were higher in patients with ICP and HCAP. Patients with ICP fulfilled more minor ATS criteria for severe pneumonia.
Severity of systemic response did not differ among the three groups of pneumonia; the overall incidences of severe sepsis and septic shock were 24 % and 50.8 % respectively (Table 2). The severity of respiratory failure was higher in the ICP group, with an incidence of ARDS of 37.2 % compared with 18 % in the CAP and HCAP (p < 0.001). There was a trend towards a higher incidence of pleural effusion in the CAP group compared to the HCAP and ICP groups (p = 0.08). Patients with HCAP or ICP needed mechanical ventilation and tracheotomy more often than patients with CAP.
Microbiology and antibiotic therapy
A positive microbiological diagnosis was made in 47.4 % of patients with HCAP compared with 56.2 % of those with CAP and 63.4 % of those with ICP (p = 0.02). Table 3 shows the frequencies of the organisms isolated in each group. The overall incidence of bacteremia was 31.3 % and there were not differences among the three groups (Electronic Supplemental Material, Table 1). S. pneumoniae was the most frequent pathogen in each of the three groups, but the incidence differed among groups (HCAP, 19.5 %; CAP, 34.2 %; and ICP, 23.4 %; p = 0.001). The ICP and HCAP groups had the highest incidence of P. aeruginosa (p = 0.08), and the HCAP group had the highest incidence of MRSA (p = 0.04). The PRMO was identified in 13 patients in the HCAP group (9.7 %), 10 in the ICP group (6.9 %), and 15 in the CAP group (3.4 %) (p = 0.001). In the logistic regression analysis, the factor most strongly associated with isolation of potentially resistant microorganisms was HCAP (OR 2.79; 95 % CI = 1.27–6.12; p = 0.01). Among 13 patients in the HCAP group with PRMO, previous hospital admission (43.1 %) and previous antibiotic therapy (38.5 %) were the most frequent risk factors. The incidence of inappropriate empirical antibiotic therapy was 6.5 % in the CAP group, 14.4 % in the HCAP group, and 21.8 % in the ICP group (p < 0.001). In the CAP group, 13.7 % of patients received inappropriate antibiotic therapy because of the isolation of PRMO (2 P. aeruginosa, 1 MRSA, 1 Klebsiella sp.); in the HCAP group, 26.3 % of patients received inappropriate antibiotic therapy because of the isolation of PRMO (2 MRSA, 1 P. aeruginosa, 1 S. maltophilia, 1 Klebsiella sp.). In the ICP group the inappropriate antibiotic therapy was not associated with the isolation of PRMO. Empirical treatment was in line with CAP guidelines (without coverage of P. aeruginosa or MRSA) in 73.5 % of patients in the CAP group; in 45.5 % of those in the HCAP group, and in 40 % of those in the ICP. Interestingly in the HCAP group, P. aeruginosa was isolated in 3 out of 54 COPD patients (5.6 %), compared to 71 non-COPD patients with only 1 isolation of P. aeruginosa isolation; however, because of the low number of cases this did not reach statistical significance (p = 0.30). In the logistic regression analysis, the factors most strongly associated with inappropriate treatment were ICP (OR 3.17; 95 % CI 1.70–5.93; p < 0.001), isolation of potentially resistant microorganisms (OR 2.50; 95 % CI 1.04–5.98; p = 0.03), and HCAP (OR 2.10; 95 % CI 1.03–4.28; p = 0.03).
Outcomes
The incidence of ICU treatment restrictions was 6.5 % in the CAP group, 12 % in the HCAP group, and 16.7 % in the ICP group (p = 0.002). The crude ICU mortality was 18.4 % in the CAP group, 21.2 % in the HCAP group, and 38.6 % in the ICP group (p < 0.001). Table 4 shows the factors significantly associated with ICU mortality in the logistic regression analysis in patients without treatment restrictions. The higher ICU mortality was significant in the ICP group, but not in the HCAP group, when both were compared with CAP group. Figure 2 shows the probabilities of survival until day 30 after the diagnosis of pneumonia.
Discussion
One-fifth of the patients admitted to our 34 ICUs with pneumonia were classified as HCAP. Mortality in patients with HCAP was not higher than in patients with CAP. Although the incidence of resistant pathogens was higher in patients with HCAP than in those with CAP, it was still low; the empirical antibiotic therapy recommended for CAP would be appropriate for 90 % of patients.
To our knowledge, this is the first study to prospectively evaluate the impact of HCAP in a large population of critically ill patients. To date, the only study published on HCAP in a large cohort of critically ill patients was a retrospective study carried out in the USA [18] to determine the frequency of multiresistant microorganisms in mechanically ventilated patients with respiratory failure. These authors reported an incidence of HCAP of nearly 50 %; however, their definition of HCAP did not exclude immunocompromised patients.
Using the ATS/IDSA definitions, which exclude immunocompromised patients, we found an incidence of HCAP of 18.3 %, which is similar to that reported by Carratala et al. [6] in another study in Spain. However, their study population included patients undergoing chemotherapy and was not limited to critically ill patients. Other studies in the general population admitted to hospital in the UK and in Italy reported similar incidences [8, 19].
By contrast, the incidences of HCAP reported in studies done in the USA are much higher [2, 3]. These differences may be partly due to the variability in the inclusion criteria for HCAP. The discrepancy between our study and those from the USA may be, in part, because our patients included in the HCAP group were younger and had less comorbid conditions. Another possible explanation might be that patients analyzed in our study were a more selected population than in previous studies, which had mostly included general ward patients admitted outside of the ICU, a substantial proportion of whom may have been denied ICU admission.
In our study, the definition of HCAP excluded immunocompromised patients, as is recommended in the guidelines of the ATS/IDSA and elsewhere [1, 2]. Analyzing patients with ICP separately showed that although the incidence ICP was similar to that of HCAP, its microbiological characteristics and prognosis were very different, supporting the idea that patients with ICP are indeed a distinct group.
In most previously published studies, mortality of patients was higher in patients with HCAP than in patients with CAP [2–4, 6, 19]. However, these studies were conducted in a general population of patients admitted to hospital. Treatment restrictions were not always recorded, but patients with HCAP were less frequently admitted to the ICU. Recently, Rello et al. [20] concluded that increased mortality in HCAP may be more attributable to comorbid conditions and limitations to aggressive intervention.
Our study population consisted of patients admitted to the ICU, so the effects of restricting ICU admission were limited, although treatment restrictions during the ICU stay were more common in patients with HCAP and those with ICP than in patients with CAP. Although the incidence of inappropriate empirical antibiotic therapy was higher in HCAP than in CAP, mortality was not significantly higher in the HCAP group. These results support the idea that mortality in HCAP is independent of bacterial susceptibility and is probably more related to age and other comorbid conditions. These results are comparable to those reported in a recent multicenter study in the UK [8], which found no difference in mortality between HCAP and CAP after excluding patients with treatment restrictions and adjusting for severity and comorbidity.
Most studies have found that patients with HCAP were older than those with CAP and had worse baseline functional status, greater comorbidities, and higher mortality [2–8]. Our results confirm these findings, although we found no significant difference in mortality. Our population consisted of critical patients who had not been denied admission to the ICU, so patients with treatment limitations prior to ICU admission were excluded a priori. Furthermore, unlike previous studies, our HCAP group excluded immunocompromised patients, and the ICP group had a significantly higher mortality. In fact, in the multivariate analysis, only immunosuppression was significantly associated with a worse prognosis.
In our study, the univariate and multivariate analyses found significantly higher mortality in the ICP group than in the other two groups, corroborating previous reports [21, 22]. Our results also reinforce the idea that this heterogeneous group of patients in whom risk varies with the diseases process should not be included among HCAP patients. It is important to consider disease-specific characteristics (febrile neutropenia in cancer, CD4 count in HIV) when making treatment decisions in patients with ICP [23], and specific guidelines include recommendations for immunocompromised patients with pneumonia [24, 25]. In the multicenter study done in the UK [8], in which the definition of HCAP also excluded immunocompromised patients, the mortality was similar to that in our study. The higher mortality in patients with HCAP in previous studies may in part be related to greater treatment restrictions and the inclusion of immunosuppressed patients.
The concept of HCAP was based on data published in 2005 [2] from a population with frequent contact with healthcare that showed a higher incidence of multidrug-resistant pathogens and therefore a greater risk of inappropriate empirical antimicrobial treatment following CAP guidelines [26]. However, more recent studies about HCAP have shown differences between countries and regions in the etiology of patients with HCAP [2–4, 6–8]. In Europe the incidence of resistant pathogens is lower, and pathogens that cause HCAP are more similar to those that cause CAP than to those that cause hospital-acquired pneumonia [26]. Chalmers et al. [8] and Grenier et al. [27] in Canada reported similar findings. These data contrast with those from the USA and the Asia/Pacific [2–5] region, where the incidences of P. aeruginosa and MRSA are much higher and S. pneumoniae accounted for less than 10 % of cases.
Our study has some limitations. First, this was an observational study, and the criteria for admission and for empiric treatment were not standardized. This may have influenced the incidence of the different types of pneumonia and empirical treatment. However, the observational nature of this study allows us to better understand the current medical prescriptions in the ICU. Second, the diagnostic techniques were not standardized and this may also have influenced the incidence of the etiologic diagnosis in the different hospitals. Third, the low incidence of MRSA in the community might reflect differences in out-of-hospital resources with other countries, and our results can not be extrapolated to other countries where Pseudomonas sp. and MRSA are more prevalent. Fourth, more than 50 % of patients with HCAP had received antibiotics within the 6 month prior to ICU admission, but we did not have information about if these patients had received the antibiotic immediately before the microbiological samples were taken. A lower number of positive results of cultures in the HCAP group may have influenced these results. Fifth, we did not have information about patients with HCAP who were denied admission to the ICU; however, these patients probably might not have had limitation of life support for ICU admission. Finally, the three groups were not matched.
In conclusion, HCAP accounted for one-fifth of cases of severe pneumonia admitted to the ICU in the present study, and did not result in a higher mortality than CAP after immunocompromised patients were excluded. The incidence of resistant pathogens in Spain is low (5.2 % in the total population), but is higher in patients with HCAP (9.7 %) than in those with CAP (3.4 %). The empirical antibiotic therapy recommended for CAP would be appropriate for 90 % of patients with HCAP in our population, and consequently the decision to include coverage of PRMO for HCAP should be cautiously judged in order to prevent the overuse of antimicrobials.
References
American Thoracic Society (2005) Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 171:388–416
Kollef MH, Shorr A, Tabak YP et al (2005) Epidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture-positive pneumonia. Chest 128:3854–3862
Micek ST, Kollef KE, Reichley RM et al (2007) Health-care-associated pneumonia and community-acquired pneumonia: a single-center experience. Antimicrob Agents Chemother 51:3568–3573
Shindo Y, Sato S, Maruyama E et al (2009) Health-care-associated pneumonia among hospitalized patients in a Japanese community hospital. Chest 135:633–640
Park HK, Song JU, Um SW et al (2010) Clinical characteristics of health care-associated pneumonia in a Korean teaching hospital. Respir Med 104:1729–1735
Carratala J, Mykietiuk A, Fernandez-Sabe N et al (2007) Health care-associated pneumonia requiring hospital admission: epidemiology, antibiotic therapy, and clinical outcomes. Arch Intern Med 167:1393–1399
Garcia-Vidal C, Viasus D, Roset A et al (2011) Low incidence of multidrug-resistant organisms in patients with healthcare-associated pneumonia requiring hospitalization. Clin Microbiol Infect 17:1659–1665
Chalmers JD, Taylor JK, Singanayagam A et al (2011) Epidemiology, antibiotic therapy, and clinical outcomes in health care-associated pneumonia: a UK cohort study. Clin Infect Dis 53:107–113
Knaus WA, Draper EA, Wagner DP et al (1985) APACHE II: a severity of disease classification system. Crit Care Med 13:818–829
Vincent JL, Moreno R, Takala J et al (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22:707–710
Fine MJ, Auble TE, Yealy DM et al (1997) A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med 336:243–250
Lim WS, van der Eerden MM, Laing R et al (2003) Defining community-acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 58:377–382
Mandell LA, Wunderink RG, Anzueto A et al (2007) Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 44(S2):S27–S72
Mahoney FI, Barthel DW (1965) Functional evaluation: the Barthel index. Md State Med J 14:61–65
Bell DM (2001) Promoting appropriate antimicrobial drug use: perspective from the Centers for Disease Control and Prevention. Clin Infect Dis 33(Suppl 3):S245–S250
Clinical and Laboratory Standards Institute (CLSI) (2011). M1000-S21. Performance standards for antimicrobial susceptibility testing: 21st Information Supplement
Bagley SC, White H, Golomb BA (2001) Logistic regression in the medical literature: standards for use and reporting, with particular attention to one medical domain. J Clin Epidemiol 54:979–985
Schreiber MP, Chan CM, Shorr AF (2010) Resistant pathogens in nonnosocomial pneumonia and respiratory failure. Is it time to refine the definition of health-care-associated pneumonia. Chest 137:1283–1288
Venditti M, Falcone M, Corrao S et al (2009) Outcomes of patients hospitalized with community-acquired, health care-associated, and hospital-acquired pneumonia. Ann Intern Med 150:19–26
Rello J, Luján M, Gallego M et al (2010) Why mortality is increased in health-care-associated pneumonia: lessons for pneumococcal bacteremic pneumonia. Chest 137:1138–1144
Sousa D, Justo I, Dominguez A et al (2013) Community-acquired pneumonia in immunocompromised older patients: incidence, causative organisms and outcome. Clin Microbiol Infect 19:187–192
de Montmollin E, Tandjaoui-Lambiotte Y, Legrand M et al (2013) Outcomes in critically ill cancer patients with septic shock of pulmonary origin. Shock 39:250–254
Attridge RT, Frei CR (2011) Health care-associated pneumonia: an evidence-based review. Am J Med 124:689–697
Kaplan JE, Benson C, Holmes KH et al (2009) Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institute of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep 58:1–207
Segal BH, Freifeld AG, Baden LR et al (2008) Prevention and treatment of cancer-related infections. J Natl Compr Canc Netw 6:122–174
Polverino E, Torres A, Menendez R et al (2013) Microbial aetiology of health care associated pneumonia (HCAP) in Spain: a prospective, multicenter, case-control study. Thorax 68:1007–1014
Grenier C, Pépin J, Nault V et al (2011) Impact of guideline-consistent therapy on outcome of patients with healthcare-associated and community-acquired pneumonia. J Antimicrob Chemother 66:1617–1624
Acknowledgments
This multicenter study was made possible owing to the effort of 34 different Spanish research groups whose collaborators all contributed to achieve the data shown in this manuscript. This study was funded entirely by the participating ICUs.
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
For the GTEI/SEMICYUC Working Group on Community-Acquired Pneumonia. A list of investigators and collaborators is given in the Appendix.
A related editorial can be found at doi:10.1007/s00134-014-3238-3.
Take-home message: HCAP does not result in higher mortality than CAP after immunocompromised patients are excluded.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix: collaborators and investigators of the Working Group on Community-Acquired Pneumonia
Appendix: collaborators and investigators of the Working Group on Community-Acquired Pneumonia
Hospital Clínico Universitario Virgen de la Arrixaca, Murcia: Dra. Enriqueta Andreu, Dr. Mario-Royo-Vilanova, Dr. Antonio Elias Martinez Pellus. Hospital General de Granollers: Dra. Diana Colon. Hospital Txagoritxu. Vitoria: Dr. Juan Fernando Castedo, Dra. Leire Larrañaga, Dr. Oscar Gutierrez. Hospital Miguel Servet. Zaragoza: Dr. Victor Gonzalez, Dr. Tomás Marsilla, Dra. Marta Gurpegui. Hospital Virgen de la Victoria. Málaga: Dra. María Nieto, Dra. Araceli Puerto. Hospital La Fe. Valencia: Dr. Juan Bonastre, Dra. Verónica Martí. Complejo Hospitalario de Pontevedra. Pontevedra: Dr. Enrique Alamparte, Dra. Ana Maria Ortega. Hospital Universitario Severo Ochoa. Leganés.Madrid: Dr. Miguel Angel Blasco, Dra. Laura Sanz. Complexo Hospitalario Universitario de Ourense. Ourense: Dra. Estrella Seoane. Hospital de Mataró. Barcelona: Dra. Mari de la Torre, Dr. Adeià Albis, Dra. Giretti Sauca, Sra. Ivana Anglade (reseach nurse). Hospital Universitario Dr. Peset. Valencia: Dr. Rafael Zaragoza, Dr. Luis Pallás, Dra. Eloína Casanoves. Hospital J.M. Morales Meseguer, Murcia: Dr. Bernardo Gil. i Dra. Eva Polverino. Hospital Universitario de Gran Canaria Dr. Negrín, Las Palmas de Gran Ganaria: Dr. Jordi Solé, Dr. José María Agüero. Hospital Universitario Joan XXIII, Tarragona: Dr. Alejandro Rodriguez, Dra.María Bodí, Sra. Mireia Llauradó (research urse). Hospital General Universitario Santa Lucía, Cartagena: Dr. Sergio Rebollo. Hospital Galdakao-Usansolo, Galdakao: Dra. Eneritz Gamboa, Dra. Celia Sañudo, Dra. Naia Mas. Hospital Universitario de Albacete, Albacete: Dr. Fernando Garcia, Dra. Miriam Gimeno. Hospital Arquitecto Marcide, Ferrol: Dra. Carmen J. Fernández, Dra. M.J. Castro. Hospital Torrecardenas, Almería: Dr. F.J. Guerrero, Dra. Josefina Moreno, Dra. Amelia Alonso. Complejo Hospitalario Universitario de Vigo, Vigo: Dr. Lucas Lage. Hospital Sabadell, Sabadell: Dra. Maria José Burgueño. Hospital Universitário de Alicante, Alicante: Dr. Bernabé Alvarez, Dr. J.Canovas. Hospital Universitário Marqués de Valdecilla, Santander: Dr. José Luis Teja, Dra. Elsa Ots. Hospital Virgen del Camino, Pamplona: Dr. Juan Angel Tihista, Dr. Enrique Maraví. Hospital Universitario Germans Trias i Pujol, Badalona: Dr. Fernando Armestar, Dra. Yariela Esther Leon. Hospital Universitario Nuestra Sra. Candelaria, Tenerife: Dra. Maria del Mar Cruz Martin. Hospital General de Burgos. Burgos: Dr. Miguel Montero. Hospital Universitario Vall d’Hebron. Barcelona: Dra. Jéssica Souto, Dra. Bárbara Borgatta, Dra. Elsa Afonso, Dr. Jordi Rello. Hospital Obispo Polanco, Teruel: Dra. Concepción Valdovinos, Dr. Jose Maria Montón, Dra. Maria Jesús Santed. Hospital Universitario Cruces, Bilbao: Dr. José Ramón Iruretagoyena, Dra. Katherine García, Dra. Ainhoa Sánchez. Hospital Universitario Virgen del Rocío, Sevilla: Dr. Antonio Gutierrez. Hospital La Paz, Madrid: Dr. Jesús Manzanares, Dra: Mónica Hernández. Hospital de Manises, Valencia: Dra. Concepción Cortés, Dr. Santiago Borrás.
Rights and permissions
About this article
Cite this article
Vallés, J., Martin-Loeches, I., Torres, A. et al. Epidemiology, antibiotic therapy and clinical outcomes of healthcare-associated pneumonia in critically ill patients: a Spanish cohort study. Intensive Care Med 40, 572–581 (2014). https://doi.org/10.1007/s00134-014-3239-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-014-3239-2