Abstract
Introduction
Missing values occur in nearly all clinical studies, despite the best efforts of the investigators, and cause frequently unrecognised biases. Our aims were (1) to assess the reporting and handling of missing values in the critical care literature; (2) to describe the impact of various techniques for handling missing values on the study results; (3) to provide guidance on the management of clinical study analysis in case of missing data.
Methods
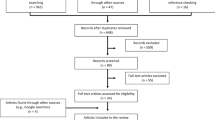
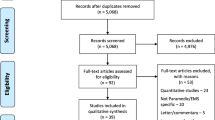
We reviewed 44 published manuscripts in three critical care research journals. We used the Conflicus study database to illustrate how to handle missing values.
Results
Among 44 published manuscripts, 16 (36.4 %) provided no information on whether missing data occurred, 6 (13.6 %) declared having no missing data, 20 (45.5 %) reported that missing values occurred but did not handle them and only 2 (4.5 %) used sophisticated missing data handling methods. In our example using the Conflicus study database, we evaluated correlations linking job strain intensity to the type and proportion of missing values. Overall, 8 % of data were missing; however, using only complete cases would have resulted in discarding 24 % of the questionnaires. A greater number and a higher percentage of missing values for a particular variable were significantly associated with a lower job strain score (indicating greater stress). Among respondents who fully completed the job strain questionnaire, the comparison of those whose questionnaires did and did not have missing values showed significant differences in terms of age, number of children and country of birth. We provided an algorithm to manage clinical studies analysis in case of missing data.
Conclusion
Missing data are common and generate interpretation biases. They should be reported routinely and taken into account when modelling data from clinical studies.


Similar content being viewed by others
References
Ibrahim JG, Chu H, Chen MH (2012) Missing data in clinical studies: issues and methods. J Clin Oncol 30:3297–3303
King G, Honaker J, Joseph A, Scheve K (2001) Analyzing incomplete political science data: an alternative algorithm for multiple imputation. Am Political Sci Rev 95:49–69
Molnar FJ, Man-Son-Hing M, Hutton B, Fergusson DA (2009) Have last-observation-carried-forward analyses caused us to favour more toxic dementia therapies over less toxic alternatives? A systematic review. Open Med 3:e31–e50
Rubin DB (1976) Inference and missing data. Biometrika 63:581–592
Touloumi G, Babiker AG, Pocock SJ, Darbyshire JH (2001) Impact of missing data due to drop-outs on estimators for rates of change in longitudinal studies: a simulation study. Stat Med 20:3715–3728
Rubin DB (1987) Multiple imputation for nonresponse in surveys. Wiley classics library, vol 81. Wiley, New York
Azoulay E, Timsit J-F, Sprung CL, Soares M, Rusinová K, Lafabrie A, Abizanda R, Svantesson M, Rubulotta F, Ricou B, Benoit D, Heyland D, Joynt G, Français A, Azeivedo-Maia P, Owczuk R, Benbenishty J, de Vita M, Valentin A, Ksomos A, Cohen S, Kompan L, Ho K, Abroug F, Kaarlola A, Gerlach H, Kyprianou T, Michalsen A, Chevret S, Schlemmer B (2009) Prevalence and factors of intensive care unit conflicts: the Conflicus study. Am J Respir Crit Care Med 180:853–860
Karasek R, Baker D, Marxer F, Ahlbom A, Theorell T (1981) Job decision latitude, job demands, and cardiovascular disease: a prospective study of Swedish men. Am J Public Health 71:694
Abayomi K, Gelman A, Levy M (2008) Diagnostics for multivariate imputations. J R Stat Soc Ser C 57:273–291
Altman DG (2009) Missing outcomes in randomized trials: addressing the dilemma. Open Med 3:e51
Little RJ, D’Agostino R, Cohen ML, Dickersin K, Emerson SS, Farrar JT, Frangakis C, Hogan JW, Molenberghs G, Murphy SA, Neaton JD, Rotnitzky A, Scharfstein D, Shih WJ, Siegel JP, Stern H (2012) The prevention and treatment of missing data in clinical trials. N Engl J Med 367:1355–1360
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vesin, A., Azoulay, E., Ruckly, S. et al. Reporting and handling missing values in clinical studies in intensive care units. Intensive Care Med 39, 1396–1404 (2013). https://doi.org/10.1007/s00134-013-2949-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-013-2949-1