Abstract
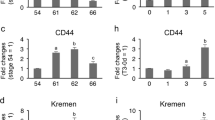
The thyroid hormone (T3)-dependent amphibian metamorphosis involves degeneration of larval tissues through programmed cell death (apoptosis) and concurrent proliferation and differentiation of adult cell types. As the mediators of the causative effects of T3 on metamorphosis, both thyroid hormone receptor (TR) α and β genes have been found to be expressed in different tissues during this process. In particular, theXenopus TRβ genes have been shown to be regulated by T3 at the transcriptional level and their expression correlates with organ-specific metamorphosis. We demonstrate here by in situ hybridization that theXenopus TRβ genes are regulated in a cell-type specific manner that correlates with tissue transformation. In particular, they are found to be expressed in the larval intestinal epithelial cells prior to their apoptotic degeneration and in the proliferating cells of the adult epithelium, connective tissue, and muscles. However, they are repressed again upon the differentiation of these adult cells. These results implicate that TRβ participates both in inducing apoptosis and stimulating cell proliferation during development.
Similar content being viewed by others
References
Brooks AR, Sweeney G, Old RW. Structure and functional expression of a cloned Xenopus thyroid hormone receptors. Nucleic Acids Res 17:9395–9405;1989.
Dauca M, Hourdry J. Transformations in the intestinal epithelium during anuran metamorphosis. In: Balls M, Bownes M, eds. Metamorphosis. Oxford, Clarendon Press, 36–58;1985.
Dodd MHI, Dodd JM. The biology of metamorphosis. In: Lofts B, ed. Physiology of the Amphibia. New York, Academic Press, 467–599;1976.
Eliceiri BP, Brown DD. Quantitation of endogenous thyroid hormone receptors α and β during embryogenesis and metamorphosis inXenopus laevis. J Biol Chem 269:24459–24465;1994.
Gilbert LI, Frieden E. Metamorphosis: A Problem in Developmental Biology, 2nd ed. New York, Plenum Press, 1981.
Gilbert LI, Tat JR, Atkinson BG. Metamorphosis: Post-Embryonic Reprogramming of Gene Expression in Amphibian and Insect Cells. New York, Academic Press, 1996.
Helbing CC, Gergely G, Atkinson BG. Sequential up-regulation of thyroid hormone β receptor, ornithine transcarbamylase, and carbamyl phosphate synthetase mRNAs in the liver ofRana catesbeiana tadpoles during spontaneous and thyroid hormone-induced metamorphosis. Dev Genet 13:289–301;1992.
Herrin DL, Schmidt GW. Rapid reversible staining of Northern blots prior to hybridization. Bio techniques 6:196–200;1988.
Ishizuya-Oka A, Shimozawa A. Development of the connective tissue in the digestive tract of the larval and metamorphosingXenopus laevis. Anat Anz 164:81–93;1987.
Ishizuya-Oka A, Shimozawa A. Programmed cell death and heterolysis of larval epithelial cells by macrophage-like cells in the anuran small intestine in vivo and in vitro. J Morphol 213:185–195;1992.
Ishizuya-Oka, Ueda S. Apoptosis and cell proliferation in theXenopus small intestine during metamorphosis. Cell Tissue Res, in press.
Kanamori A, Brown DD. The regulation of thyroid hormone receptor β genes by thyroid hormone inXenopus laevis. Biol Chem 267:739–745;1992.
Kawahara A, Baler BS, Tata JR. Developmental and regional expression of thyroid hormone receptor genes during Xenopus metamorphosis. Development 112:933–943;1991.
Kordylewski L. Light and electron microscopic observations of the development of intestinal musculature in Xenopus. Anat Forsch 97:719–734;1983.
Leloup J, Buscaglia M. La triiodothyronine: hormone de la métamorphose des amphibiens. CR Acad Sci 284:2261–2263;1977.
McAvoy JW, Dixon KE. Cell proliferation and renewal in the small intestinal epithelium of metamorphosing and adultXenopus laevis. J Exp Zool 202:129–138;1977.
Machuca I, Esslemont G, Fairclough L, Tata JR. Analysis of structure and expression of theXenopus thyroid hormone receptor β gene to explain its autoregulation. Mol Endocrinol 9:96–107;1995.
Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: The second decade. Cell 83:835–839;1995.
Maniatis T, Fritsch EF, Sambrook J. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, Cold Spring Harbor Laboratory Press, 1982.
Marshall JA, Dixon KE. Cell specialization in the epithelium of the small intestine of feedingXenopus laevis. J Anat 126:133–144;1978.
Nieuwkoop PD, Faber J. Normal Table ofXenopus laevis. Amsterdam, North Holland, 1956.
Ranjan M, Wong J, Shi Y-B. Transcriptional repression of Xenopus TRβ gene is mediated by a thyroid hormone response element located near the start site. J Biol Chem 269:24699–24705;1994.
Schneider MJ, Galton VA. Regulation of c-erbA-α messenger RNA species in tadpole erythrocytes by thyroid hormone. Mol Endocrinol 5:201–208;1991.
Shi Y-B, Liang VC-T. Cloning and characterization of the ribosomal protein L8 gene fromXenopus laevis. Biochim Biophys Acta 1217:227–228;1994.
Shi Y-B, Liang VC-T, Parkison C, Cheng S-Y. Tissue-dependent developmental expression of a cytosolic thyroid hormone protein gene in Xenopus: Its role in the regulation of amphibian metamorphosis. FEBS Lett 355:61–64;1994.
Shi Y-B, Hayes WP. Thyroid hormone-dependent regulation of the intestinal fatty acid-binding protein gene during amphibian metamorphosis. Dev Biol 161:48–58;1994.
Shi Y-B. Molecular biology of amphibian metamorphosis: A new approach to an old problem. Trends Endocrinol Metab 5:14–20;1994.
Shi Y-B, Ishizuya-Oka A. Biphasic intestinal development in amphibians: Embryogenesis and remodeling during metamorphosis. Curr Top Dev Biol 32:205–235;1996.
Tsai M-J, O'Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem 63:451–486;1994.
Wang Z, Brown DD. Thyroid hormone-induced gene expression program for amphibian tail resorption. J Biol Chem 268:16270–16278;1993.
Wong J, Shi Y-B, Wolffe AP. A role for nucleosome assembly in both silencing and activation of the Xenopus TRβA gene by the thyroid hormone receptor. Genes Dev 9:2696–2711;1995.
Wong J, Shi Y-B. Coordinated regulation of and transcriptional activation byXenopus thyroid hormone and retinoid X receptors. J Biol Chem 270:18479–18483;1995.
Yaoita Y, Shi Y-B, Brown DD.Xenopus laevis α and β thyroid hormone receptors. Proc Natl Acad Sci USA 87:7090–7094;1990.
Yaoita Y, Brown DD. A correlation of thyroid hormone receptor gene expression with amphibian metamorphosis. Gene Dev 4:1917–1924;1990.
Yen PM, Chin WW. New advances in understanding the molecular mechanisms of thyroid hormone action. Trends Endocrinol Metab 5:65–72;1994.
Yoshizato K. Biochemistry and cell biology of amphibian metamorphosis with a special emphasis on the mechanism of removal of larval organs. Int Rev Cytol 119:97–149;1989.
Shi YB, Wong J, Puzianowska-Kuznicka M. Thyroid hormone receptors: Mechanisms of transcriptional regulation and roles during frog development. J Biomed Sci 3:307–318;1996.
Ishizuya-Oka A, Shimozawa A. Connective tissue is involved in adult epithelial development of the small intestine during anuran metamorphosis in vitro. Rouxs Arch Dev Biol 201:322–329;1992.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shi, YB., Ishizuya-Oka, A. Autoactivation ofXenopus thyroid hormone receptor β genes correlates with larval epithelial apoptosis and adult cell proliferation. J Biomed Sci 4, 9–18 (1997). https://doi.org/10.1007/BF02255588
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02255588