Article Text
Abstract
Background In allergic asthma, exposure to relevant antigens leads to an early asthmatic response (EAR) followed, in certain subjects, by a late asthmatic response (LAR). Although many subjects with asthma consider LAR to be one of the defining symptoms of their disease, and despite its widespread use in the clinical assessment of new therapeutic entities, the mechanism underlying the LAR remains unclear.
Method A study was undertaken using ovalbumin-sensitised and challenged Brown Norway rat and C57BL/6J mouse models which recapitulate phenotypic features of allergic asthma including the LAR and its susceptibility to clinically effective agents.
Results In conscious animals an EAR was followed by a LAR. The LAR was subjectively evidenced by audible (wheeze) and visual signs of respiratory distress associated with quantifiable changes in non-invasive lung function assessment. Treatments that attenuated the EAR failed to impact on the LAR and, while anaesthesia did not impact on EAR, it abolished LAR. A key role for airway sensory neuronal reflexes in the LAR was therefore hypothesised, which was confirmed by the blockade observed after administration of ruthenium red (non-selective cation channel blocker), HC-030031 (TRPA1 inhibitor) and tiotropium bromide (anticholinergic) but not JNJ-17203212 (TRPV1 inhibitor).
Conclusion These results suggest that LAR involves the following processes: allergen challenge triggering airway sensory nerves via the activation of TRPA1 channels which initiates a central reflex event leading to a parasympathetic cholinergic constrictor response. These data are supported by recent clinical trials suggesting that an anticholinergic agent improved symptoms and lung function in patients with asthma.
- Ovalbumin
- sensory nerves
- late asthmatic response
- COPD mechanisms
- asthma
- asthma mechanisms
- asthma pharmacology
- COPD pathology
- COPD pharmacology
- emphysema
- COPD exacerbations
- cough/mechanisms/pharmacology
- respiratory infection
- viral infection
- airway epithelium
- asthma epidemiology
- cytokine biology
- eosinophil biology
- exhaled airway markers
- lung physiology
- allergic lung disease
- asthma guidelines
- allergic lung disease
- paediatric asthma
Statistics from Altmetric.com
- Ovalbumin
- sensory nerves
- late asthmatic response
- COPD mechanisms
- asthma
- asthma mechanisms
- asthma pharmacology
- COPD pathology
- COPD pharmacology
- emphysema
- COPD exacerbations
- cough/mechanisms/pharmacology
- respiratory infection
- viral infection
- airway epithelium
- asthma epidemiology
- cytokine biology
- eosinophil biology
- exhaled airway markers
- lung physiology
- allergic lung disease
- asthma guidelines
- allergic lung disease
- paediatric asthma
Key messages
What is the key question?
Although many asthmatics consider the late asthmatic response (LAR) to be one of the defining symptoms of their disease and despite its widespread use in the clinical assessment of new therapeutic entities, the mechanism underlying the LAR remains unclear.
What is the bottom line?
Our results suggest that LAR involves allergen challenge triggering airway sensory nerves via the activation of TRPA1 channels which initiates a central reflex event leading to a parasympathetic, cholinergic constrictor response.
Why read on?
This data may provide a mechanistic explanation for positive data in recent clinical trials suggesting that an anti-cholinergic improved symptoms and lung function in asthma patients.
Introduction
Asthma is a chronic inflammatory disease of the airways that is characterised by variable airflow obstruction and airway hyper-responsiveness (AHR) and the presence of symptoms such as dyspnoea, chest tightness, wheezing and cough.1 According to the World Health Organization, an estimated 300 million people have asthma, representing a major public health issue, with an increasing prevalence and associated mortality.1 2
Epidemiological studies show environmental allergens (eg, pollens and house dust mite) to be important inducers of asthma.3 Allergen inhalational challenge via the aerosol delivery of specific allergens in patients with mild asthma has been well characterised, is a useful model for understanding the mechanisms involved in the pathophysiology of asthma4 5 and has been predictive of therapeutic utility.6 7 Allergen inhalation by allergic subjects results in a bronchoconstrictor response which is characterised by two phases and is associated with AHR and eosinophilic inflammation.8 The acute or early bronchoconstrictor response (EAR) occurs within minutes after allergen exposure and is associated with the allergen causing cross-linking of the IgE on mast cells leading to mast cell degranulation and the release of inflammatory mediators such as histamine and cysteinyl-leukotrienes. Approximately 50% of subjects with asthma with an EAR will experience a late asthmatic response (LAR) which follows the EAR 3–8 h after allergen exposure.9
The current view is that allergen-induced LAR is thought to involve eosinophilic airway inflammation and a subsequent increase in oedema. Supporting this hypothesis is the fact that studies using clinically effective doses of inhaled steroids have shown marked inhibition of the LAR10–13 and eosinophil influx,13 and this has also been observed with other effective agents including omalizumab14 and β2 agonists.7 15 However, the precise mechanisms underlying this response are still unclear and controversial. Elucidating the nature of the LAR remains an intriguing challenge, given the use of these models in the discovery of novel therapeutic strategies for disease treatment.
The late sequelae following allergen challenge can be recapitulated in animal models16–23 including the Brown Norway rat20 22 and the C57BL/6J mouse.17 Furthermore, currently approved drugs for asthma (including β2 agonists and steroids) modify this response.22 23 These animal models are characterised by inflammatory infiltration and a biphasic bronchoconstrictor response (EAR and LAR) to allergen challenge with audible (wheeze) and visual signs of respiratory distress associated with quantifiable changes in non-invasive lung function assessment.22 In this paper we suggest, for the first time, that airway sensory nerves, central reflexes and a parasympathetic effector arm leading to cholinergic constrictor responses play a key role in the LAR response in these preclinical models. In order to study this intact autonomic effector mechanism over protracted periods of time, we chose specifically to use an indicator of lung function, enhanced pause (Penh), which has previously been used to assess lung function in awake animals thus preserving the neurological output of the CNS.
Methods
Animals
Male Brown Norway rats (200–225 g) and C57BL/6J mice were obtained from Harlan (UK). The animals were housed for 1 week before use. Food and water were supplied ad libitum. Experiments were performed in accordance with the UK Home Office guidelines for animal welfare based on the Animals (Scientific Procedures) Act 1986.
Sensitisation and challenge
Brown Norway rats were sensitised and challenged with ovalbumin (OVA, Sigma, UK) or saline as described previously.22 C57BL/6J mice were sensitised and challenged with a single OVA exposure as described previously.17
Demonstration in conscious animals of EAR and LAR after inhaled OVA
On day 28, conscious and unrestrained saline- or OVA-sensitised rats were placed in a whole body plethysmograph (WBP) and pressure changes measured as previously described.22 Penh was recorded for 2 min (baseline) before the rats were exposed to aerosolised OVA for 20 min. Mean Penh values were recorded for up to 6 h after challenge. Owing to the times of the pharmacological interventions, OVA challenge in mice and rats in subsequent experiments was carried out outside the WBP chamber via aerosol challenge in a Perspex chamber (rat) or by intratracheal administration under isoflurane anaesthesia (mice) and Penh was recorded for 1–6 h after challenge.
Effect of anaesthesia on LAR
Several symptoms of asthma such as cough, wheezing and dyspnoea are thought to be under neural control. To investigate the role of the CNS in the allergen-induced LAR, the following experiments were performed. The effect of anaesthesia was examined in Brown Norway rats on established OVA-induced LAR. Conscious OVA-sensitised rats were challenged with aerosolised saline or OVA (1% w/v in saline) as described above. The presence of LAR was observed and monitored (visual and audible signs of respiratory distress) as above. Once the LAR was established, the conscious rats were anaesthetised with intraperitoneal ketamine and xylazine (144 and 10 mg/kg, respectively), mechanically ventilated and cannulated as described previously24 and changes in resistance were determined. In a separate experiment, naïve rats were anaesthetised with intraperitoneal ketamine and xylazine (144 and 10 mg/kg, respectively) and instrumented as above, then exposed to inhaled methacholine (16 mg/ml) and changes in resistance recorded.
Pharmacological modulation of OVA-induced LAR
On day 28, OVA-sensitised rats were challenged in a Perspex chamber with vehicle (saline, aerosolised by a nebuliser for 30 min) or OVA (1% w/v in saline). Before and after challenge the rats received the appropriate vehicle or budesonide (3 mg/kg orally), formoterol (2 mg/kg orally), methysergide (10 mg/kg intraperitoneally), montelukast (30 mg/kg orally), mepyramine (10 mg/kg intraperitoneally), ruthenium red (2 mg/kg intraperitoneally), JNJ-17203212 (100mg/kg intraperitoneally), HC-030031 (30-100-300 mg/kg intraperitoneally) or tiotropium bromide (0.1 mg/kg intratracheally).22 24–28 One hour after the challenge the LAR was monitored for up to 5 h.
On day 28, OVA-sensitised mice were challenged with vehicle (intratracheal saline) or OVA (intratracheal 2% w/v in saline). Before challenge the mice received the appropriate vehicle or budesonide (3 mg/kg orally), ruthenium red (2 mg/kg intraperitoneally), JNJ-17203212 (100 mg/kg intraperitoneally), HC-030031 (300 mg/kg intraperitoneally) or tiotropium bromide (0.02 mg/kg intranasally). One hour after the challenge the LAR was monitored for up to 5 h. All details are given in the figure legends.
Drugs
Methysergide was donated by GlaxoSmithKline Pharmaceuticals, Stevenage, UK. Mepyramine was obtained from Rhone-Poulenc Rorer Ltd, Dagenham, UK; montelukast from Cayman Chemical, Ann Arbor, USA; capsaicin, budesonide, ruthenium red and formoterol from Sigma-Aldrich, Poole, UK; HC-030031 from Chembridge, San Diego, USA; tiotropium bromide from Kemprotec, Middlesbrough, UK; and JNJ-17203212 from Tocris, Bristol, UK.
Results
Demonstration of EAR and LAR after inhaled OVA
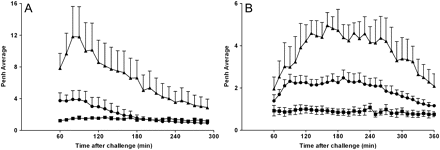
Exposure to inhaled OVA caused an increase in audible (wheeze) and visual signs of respiratory distress which were measured subjectively and correlated temporally with objective measurements (Penh) in all rats and mice previously sensitised to the antigen but not the vehicle. The sensitised and challenged rats had both an EAR and a LAR (figure 1A). The LAR could also be observed in mice (figure 1C). Treatment with approved clinically effective asthma therapies—a β2 adrenoceptor agonist (formoterol) or a glucocorticosteroid (budesonide)—attenuated the LAR, suggesting that parallels exist between humans and animal models with regard to the susceptibility to currently used therapeutics (figure 1B,C). As β1 and not β2 adrenoceptors mediate β agonist bronchodilator activity in mouse airways, formoterol was not tested in the mouse asthma model.29
Early asthmatic response (EAR) and late asthmatic response (LAR) after inhaled ovalbumin (OVA) challenge in sensitised conscious Brown Norway rats, C57BL/6J mice and the effect of gold standard asthma therapies. (A) Intraperitoneal saline-sensitised (square) or OVA-sensitised (triangle) Brown Norway rats were exposed to aerosolised OVA (20 min, 5% w/v in saline) in whole body plethysmography chambers while EAR and LAR were monitored by observation (audible and visual signs) and changes in enhanced pause (Penh). Data represent mean±SEM Penh (n=4). (B) OVA-sensitised rats received oral vehicle (0.5% methylcellulose and 0.2% Tween80 in H2O (square and triangle), budesonide (3 mg/kg; circle) or formoterol (2 mg/kg; diamond) 1 h before and 1 h after challenge. Rats were challenged with aerosolised saline (square) or OVA (diamond, circle and triangle, 1% w/v in saline) for 30 min and the LAR was monitored for 1–6 h after challenge. Data represent mean±SEM Penh (n=5–8). (C) Saline-sensitised (square) or OVA-sensitised (triangle and circle) C57BL/6J mice received oral vehicle (0.5% methylcellulose and 0.2% Tween80 in H2O; square and triangle) or budesonide (3 mg/kg, circle) 60 min prior to OVA challenge (25 μl intratracheally, 2% w/v in saline). The LAR was monitored for 1–6 h after challenge. Data represent mean±SEM Penh (n=5–6).
Effect of mepyramine, methysergide and montelukast on the LAR following OVA challenge
Previously published data have shown that methysergide and montelukast treatment blocked the EAR, which suggests that this allergic response is driven by 5-hydroxytryptamine (5-HT) and cysteinyl-leukotrienes.24 Treatment with mepyramine, methysergide and montelukast individually (data not shown) or together (figure 2) failed to have a marked impact on the LAR despite their effect on EAR. None of the compounds tested had any effect on baseline readings (data not shown). This suggests that those mediators that are involved in the EAR are not driving the LAR, and that the LAR can develop irrespective of the presence of an EAR.
Effect of mepyramine, methysergide and montelukast on ovalbumin (OVA)-induced late asthmatic response. Saline-sensitised (square) or OVA-sensitised (triangle and circle) Brown Norway rats were challenged with aerosolised OVA (1% w/v in saline) for 30 min. Animals received intraperitoneal methysergide (10 mg/kg) and mepyramine (10 mg/kg) 30 min before and after challenge and oral montelukast (30 mg/kg; circle) 90 min before and 60 min after OVA challenge or the appropriate vehicle at the corresponding time points (intraperitoneal saline and oral 0.5% methylcellulose and 0.2% Tween80 in H2O; square and triangle). The enhanced pause (Penh) was recorded for 1–6 h after challenge. Data are presented as mean±SEM Penh (n=8).
Modulation of the LAR following OVA challenge by inhibition of sensory nerve activation
Conscious rats were exposed to aerosolised OVA and developed a robust LAR compared with animals exposed to saline. These same animals, with an established LAR, were anaesthetised with ketamine and xylazine and intubated and ventilated for resistance measurements. Animals that had exhibited a LAR while conscious lost this response after anaesthesia. Indeed, no discernable differences in resistance were measured between saline-challenged and OVA-challenged animals after anaesthesia, even though it was shown that a LAR was present in these animals both by Penh measurements and by observations of audible and visual signs of respiratory distress. These data indicate a possible role for a central neuronal reflex component to the OVA-induced LAR in this model, although an effect of anaesthesia on peripheral sensory nerves cannot be ruled out. Further evidence in support of sensory nerve activation and a central reflex component to the LAR is that ruthenium red, a non-selective transient receptor potential (TRP) blocker, attenuates the LAR both in rats and mice (figure 3A,B respectively). These data support the hypothesis that OVA challenge can activate sensory nerves resulting in a central neural reflex and ultimately smooth muscle contraction and the resulting LAR. In subsequent experiments we investigated the role of two cation channels of the TRP family, TRPV1 and TRPA1, which have been shown to be involved in the initiation of airway sensory reflexes and OVA-induced airway inflammatory responses.30–34 JNJ-17203212, a TRPV1 blocker, inhibited capsaicin-induced airway constriction in both rats and mice (figure 4A,B; inset graphs). JNJ-17203212, however, was not able to affect the OVA-induced LAR in the rat or mouse asthma model (figure 4A,B). Similarly, capsazepine, another TRPV1 blocker, did not reduce the allergen-induced airway responses in these models (data not shown). In contrast, the TRPA1 blocker HC-030031 inhibited OVA-induced LAR in both the rat (figure 4A) and murine asthma model (figure 4B). None of the compounds tested had any effect on baseline readings (data not shown). These data indicate a role for TRPA1 and not TRPV1 in allergen-induced LAR in these two species.
Effect of ruthenium red on ovalbumin (OVA)-induced late asthmatic response. (A) OVA-sensitised Brown Norway rats were treated with intraperitoneal ruthenium red (2 mg/kg; circle) or vehicle (saline; triangle and square) 1 h before and 1 h after exposure to aerosolised saline (square) or OVA (1% w/v in saline; triangle and circle). The enhanced pause (Penh) was recorded for 1–6 h after challenge. Data are presented as mean±SEM Penh (n=5–8). (B) OVA-sensitised C57BL/6J mice were challenged with intracheal OVA (25 μl, 2% w/v in saline). One hour before challenge the animals received intraperitoneal ruthenium red (2 mg/kg; circle) or vehicle (0.5% methylcellulose and 0.2% Tween80 in saline; triangle). For comparison, the saline-sensitised and OVA-challenged control group (square) from figure 1C was added to this graph. Penh was recorded for 1–6 h after challenge. Data are presented as mean±SEM Penh (n=5–7).
Involvement of TRPV1 and TRPA1 in ovalbumin (OVA)-induced late asthmatic response. (A) OVA-sensitised Brown Norway rats were treated with intraperitoneal vehicle (0.5% methylcellulose and 0.2% Tween80 in saline; triangle and square), HC-030031 (30 (reverse triangle)-100 (open circle)-300 (closed circle) mg/kg) or JNJ-17203212 (100 mg/kg; diamond) 120 min before and 30 min after challenge with aerosolised saline (square) or OVA (1% w/v in saline) (open circle, closed circle, reverse triangle, triangle). The enhanced pause (Penh) was recorded for 1–6 h after challenge. Data are presented as mean±SEM Penh (n=7–8). The inset graph shows the inhibitory effect of JNJ-17203212 on capsaicin-induced increases in Penh. Naïve rats were pretreated with intraperitoneal JNJ-17203212 (100 mg/kg; closed bar) or vehicle (0.5% methylcellulose and 0.2% Tween80 in saline; open bar) 1 h before exposure to aerosolised capsaicin (10 μM). Penh was recorded for up to 10 min after aerosol exposure. Data are presented as the mean ±SEM increase in Penh area under the curve (AUC) compared with saline control aerosol (n=6). (B) OVA-sensitised C57BL/6J mice were challenged with intratracheal OVA (25 μl, 2% w/v in saline). One hour before challenge the animals received intraperitoneal HC-030031 (300 mg/kg; circle), JNJ-17203212 (100 mg/kg; diamond) or vehicle (0.5% methylcellulose and 0.2% Tween80 in saline; triangle). For comparison, the saline-sensitised and OVA-challenged control group (square) from figure 1C was added to this graph. Penh was recorded for 1–6 h after challenge. Data are presented as mean ±SEM Penh (n=5–7). The inset graph shows the inhibitory effect of JNJ-17203212 on capsaicin-induced increases in Penh. Naïve mice were pretreated with intraperitoneal JNJ-17203212 (100 mg/kg; closed bar) or vehicle (0.5% methylcellulose and 0.2% Tween80 in saline; open bar) 1 h before exposure to aerosolised capsaicin (10 μM). Penh was recorded for up to 10 min after aerosol exposure. Data are presented as the mean ±SEM increase in Penh AUC compared with saline control aerosol (n=6). Statistical significance of the data was assessed using the unpaired t test for parametric data. A p value of <0.05 was taken as significant.
Modulation of the LAR following OVA challenge by a long-acting anticholinergic agent
Tiotropium inhibited methacholine-induced bronchospasm in conscious rats (data not shown). The same dose significantly reduced the OVA-induced LAR (figure 5A). Similarly to the rat OVA model, tiotropium inhibited methacholine-induced bronchospasm in conscious mice (data not shown) and attenuated the OVA-induced LAR in the mouse (figure 5B). These data reinforce the proposed mechanism of action and suggest that, following OVA challenge, airway sensory nerves can be activated and drive central reflexes and a parasympathetic effector arm leading to cholinergic constrictor responses and the resulting LAR (figure 6).
Effect of tiotropium bromide on the ovalbumin (OVA)-induced late asthmatic response. (A) OVA-sensitised Brown Norway rats were treated with intratracheal vehicle (0.5% ethanol in saline; triangle and square) or tiotropium bromide (0.1 mg/kg; circle) 30 min before and after challenge with aerosolised saline (square) or OVA (1% w/v in saline; circle and triangle). The enhanced pause (Penh) was recorded for 1–5 h after challenge. Data are presented as mean±SEM Penh (n=6–7). (B) OVA-sensitised C57BL/6J mice were challenged with intratracheal OVA (25 μl, 2% w/v in saline). One hour before the challenge the animals received intranasal tiotropium (50 μl, 0.02 mg/kg; circle) or vehicle (0.5% ethanol in saline; triangle). For comparison, the saline-sensitised and OVA-challenged control group (square) from figure 1C was added to this graph. Penh was recorded for 1–6 h after challenge. Data are presented as mean±SEM Penh (n=3–5).
Schematic representation of the proposed mechanisms involved in the late asthmatic response (LAR). Allergen challenge leads to sensory nerve activation via the TRPA1 channel (blocked by ruthenium red, HC-030031) and central reflex events (blocked by anaesthesia) ultimately leading to a cholinergic reflex bronchoconstrictor response (blocked by tiotropium) which may be responsible for the LAR seen in this model.
Discussion
The current view is that allergen-induced LAR is thought to involve T cell activation and eosinophilic airway inflammation and a subsequent increase in oedema. Supporting this hypothesis is the fact that studies using clinically effective doses of inhaled steroids have shown marked inhibition of the LAR10–13 and eosinophil influx.13 However, despite extensive research, the precise mechanisms underlying this response are still unclear and controversial. Elucidating the nature of the LAR remains an exciting challenge given the use of these models in the discovery of novel therapeutic strategies for disease treatment.
In this study we have used two preclinical in vivo models (rat and mouse) to determine the mechanism driving the LAR. We have repeated previous findings demonstrating that the EAR, but not the LAR, is driven by the mast cell mediators 5-HT and cysteinyl-leukotrienes,24 and that blocking the EAR does not impact on the LAR. In subjects with asthma who exhibit a dual bronchoconstrictor response to allergen challenge, the EAR occurs within the first hour after challenge. The second response, the LAR, occurs 3–8 h after challenge and can last up to 24 h.9 Although animal models can exhibit similar dual responses, often the timeframe in which they develop differs from the clinical situation. For instance, allergen exposure in guinea pigs can lead to an EAR and a LAR but, while the EAR may last up to 6 h after challenge, the LAR occurs 7–11 h after challenge.35 In an allergic murine model of asthma, allergen exposure resulted in an EAR up to 2 h after challenge while the LAR was present from 2 h to >8 h after challenge.17 In the model described in this study, the EAR occurs within the first 20 min after challenge and a LAR is observed 1–6 h after challenge. Clearly there are differences between the clinical situation and the kinetics of the EAR and LAR development in response to allergen challenge in animal models. However, the EAR and LAR shown in the present models, like in human asthma, can be distinguished by using various pharmacological interventions. Indeed, as in the clinic, a single dose of steroid was able to block the LAR while there was no effect on the EAR.
The LAR in these models is evidenced as an audible (wheeze) and visual signs of respiratory distress (assessed subjectively) which were quantifiable by changes in non-invasive lung function assessment in conscious animals. Many other stimuli (eg, exercise, cold air, distilled water) that can provoke asthma-type symptoms cause bronchoconstriction that may be due, at least in part, to reflex bronchoconstriction mediated through activation of the parasympathetic nerve pathway.36–38 Interestingly, in this study animals that had exhibited a LAR while conscious lost this response following anaesthesia. This suggests that the LAR may also involve the generation of central reflex events.
We have shown that inhaled OVA caused an increase in Penh which is measured by non-invasive WBP in OVA-sensitised rats and mice but not in animals sensitised to saline. These measurements correlated temporally with subjective assessments including an increase in audible wheeze and visual signs of respiratory distress such as lack of activity and gasping for breath, which indicated that we were not measuring changes in the upper airway (ie, changes in airflow obstruction in the nose) although this possibility cannot be completely discounted. Furthermore, when allergen was delivered intratracheally directly into the mouse airways, a LAR was evident which also suggests that Penh is detecting changes in the lung rather than the upper airway/nose, although this cannot be completely ruled out. Although several researchers have reported on and used WBP and the Penh parameter for many years as an indicator of airflow obstruction,21 39–42 some recent publications have argued against the use of Penh as a measure of bronchoconstriction and have urged caution in the interpretation of such data.43 Similarly, there is an increasing demand to back up any recording performed in conscious animals with airway resistance measurements in anaesthetised, ventilated and instrumented animals. While we are in agreement with these concerns, it is clear from the data that anaesthesia abolishes the functional phenotype we are attempting to model. Currently, there are very few options with regard to the measurement of lung mechanics in conscious small laboratory rodents and, of those available, it would not be possible to restrain animals for the periods of time required to measure the LAR (up to 5 h). Hence we have used WBP with all the caveats discussed in the publications mentioned.
Further evidence for a neuronal component to the LAR was the marked blockade observed after administration of the non-specific cation channel blocker ruthenium red in both species.25 26 This finding supports the hypothesis that OVA challenge can activate sensory nerves resulting in a central neural reflex and ultimately smooth muscle contraction. In subsequent experiments we specifically investigated the role of two cation channels of the TRP family, TRPV1 and TRPA1, which have been shown to be involved in the initiation of airway sensory reflexes and OVA-induced airway inflammatory responses.30–34 Interestingly, the TRPV1 blocker JNJ-17203212 inhibited capsaicin-induced bronchospasm but was not able to inhibit LAR. In contrast, the TRPA1 blocker HC-030031 inhibited OVA-induced LAR in a dose-related fashion. A further data set describing the inhibitory action of the clinically used anticholinergic compound tiotropium on the OVA-induced LAR in both species provides extra support for a mechanism involving allergen-induced release of an endogenous TRPA1 activator which stimulates airway sensory nerves and a central reflex which ultimately leads to cholinergic bronchospasm.
It is not yet clear how allergen activates the TRPA1 channel, but it is likely that it stimulates the channel indirectly via the release of endogenously-produced TRPA1 activators. Interestingly, inflammation can lead to the formation of endogenous electrophilic compounds in vivo. α,β-unsaturated aldehydes have been detected in increased levels in patients with asthma and are known to activate TRPA1.44 Other examples of such compounds are the cyclopentenone ring-containing A- and J-series prostaglandins which are formed as non-enzymatic dehydration products of PGE2 and PGD2, respectively. Prostanoids that contain one or two electrophilic carbons such as 15PGJ2, ∆12-PGJ2, 8-iso PGA2 and PGA2 are therefore able to activate nociceptive neurons via direct interaction with TRPA1.45 Furthermore, prostaglandins such as PGE2 which can indirectly activate TRPA1 following activation of the EP3 receptor46 and the inducible form of cyclooxygenase (COX-2) are elevated in respiratory disease states at the site of inflammation.47 48 In addition, bradykinin has also been implicated as a cause of airway responses to eosinophilic cation proteins in rats49 and may act in part through TRPA1 activation.50 Taken together, this information would suggest that reactive prostanoids and other endogenous TRPA1 ligands, which are produced in greater amounts during inflammation or oxidant stress, could evoke the LAR seen in asthma. Interestingly, toluene di-isocyanate (TDI), a reactive compound extensively used in the manufacture of polymeric derivatives, can cause respiratory symptoms including LAR in exposed workers.51 Recent data suggest that the TRPA1 receptor mediates TDI-induced respiratory irritation, suggesting that in this case the injurious agent could directly stimulate sensory nerves to initiate the LAR.52
In conclusion, this study has, for the first time, elucidated the mechanisms involved in the LAR. The results of this study suggest that airway sensory nerves, central reflexes and a parasympathetic effector arm leading to cholinergic constrictor responses play a key role in the LAR response in two preclinical models in different species (figure 6). This finding, if reproduced in patients with asthma, suggests that anticholinergic therapy may be effective in patients with a late phase response to allergen. The data are supported by recent clinical studies suggesting that an anticholinergic agent improved symptoms and lung function in patients with asthma.53
References
Footnotes
Funding The project was funded by the Medical Research Council (MRC), UK. MAB, SAM and MG were funded by project grants from the MRC (MAB, MG, G0800196; SAM, G0800195).
Competing interests None.
Provenance and peer review Not commissioned; externally peer reviewed.
Linked Articles
- Airwaves