-
PDF
- Split View
-
Views
-
Cite
Cite
Stavros V. Konstantinides, Adam Torbicki, Giancarlo Agnelli, Nicolas Danchin, David Fitzmaurice, Nazzareno Galiè, J. Simon R. Gibbs, Menno V. Huisman, Marc Humbert, Nils Kucher, Irene Lang, Mareike Lankeit, John Lekakis, Christoph Maack, Eckhard Mayer, Nicolas Meneveau, Arnaud Perrier, Piotr Pruszczyk, Lars H. Rasmussen, Thomas H. Schindler, Pavel Svitil, Anton Vonk Noordegraaf, Jose Luis Zamorano, Maurizio Zompatori, Jose Luis Zamorano, Stephan Achenbach, Helmut Baumgartner, Jeroen J. Bax, Hector Bueno, Veronica Dean, Christi Deaton, Çetin Erol, Robert Fagard, Roberto Ferrari, David Hasdai, Arno Hoes, Paulus Kirchhof, Juhani Knuuti, Philippe Kolh, Patrizio Lancellotti, Ales Linhart, Petros Nihoyannopoulos, Massimo F. Piepoli, Piotr Ponikowski, Per Anton Sirnes, Juan Luis Tamargo, Michal Tendera, Adam Torbicki, William Wijns, Stephan Windecker, Çetin Erol, David Jimenez, Walter Ageno, Stefan Agewall, Riccardo Asteggiano, Rupert Bauersachs, Cecilia Becattini, Henri Bounameaux, Harry R. Büller, Constantinos H. Davos, Christi Deaton, Geert-Jan Geersing, Miguel Angel Gómez Sanchez, Jeroen Hendriks, Arno Hoes, Mustafa Kilickap, Viacheslav Mareev, Manuel Monreal, Joao Morais, Petros Nihoyannopoulos, Bogdan A. Popescu, Olivier Sanchez, Alex C. Spyropoulos, 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism: The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC)
Endorsed by the European Respiratory Society (ERS), European Heart Journal, Volume 35, Issue 43, 14 November 2014, Pages 3033–3080, https://doi.org/10.1093/eurheartj/ehu283Close - Share Icon Share
Abbreviations and acronyms
- ACS
acute coronary syndrome
- AMPLIFY
Apixaban for the Initial Management of Pulmonary Embolism and Deep-Vein Thrombosis as First-line Therapy
- aPTT
activated partial thromboplastin time
- b.i.d.
bis in diem (twice daily)
- b.p.m.
beats per minute
- BNP
brain natriuretic peptide
- BP
blood pressure
- CI
confidence interval
- CO
cardiac output
- COPD
chronic obstructive pulmonary disease
- CPG
Committee for Practice Guidelines
- CRNM
clinically relevant non-major
- CT
computed tomographic/tomogram
- CTEPH
chronic thromboembolic pulmonary hypertension
- CUS
compression venous ultrasonography
- DSA
digital subtraction angiography
- DVT
deep vein thrombosis
- ELISA
enzyme-linked immunosorbent assay
- ESC
European Society of Cardiology
- H-FABP
heart-type fatty acid-binding protein
- HIT
heparin-induced thrombocytopenia
- HR
hazard ratio
- ICOPER
International Cooperative Pulmonary Embolism Registry
- ICRP
International Commission on Radiological Protection
- INR
international normalized ratio
- iPAH
idiopathic pulmonary arterial hypertension
- IVC
inferior vena cava
- LMWH
low molecular weight heparin
- LV
left ventricle/left ventricular
- MDCT
multi-detector computed tomographic (angiography)
- MRA
magnetic resonance angiography
- NGAL
neutrophil gelatinase-associated lipocalin
- NOAC(s)
Non-vitamin K-dependent new oral anticoagulant(s)
- NT-proBNP
N-terminal pro-brain natriuretic peptide
- o.d.
omni die (every day)
- OR
odds ratio
- PAH
pulmonary arterial hypertension
- PE
pulmonary embolism
- PEA
pulmonary endarterectomy
- PEITHO
Pulmonary EmbolIsm THrOmbolysis trial
- PESI
pulmonary embolism severity index
- PH
pulmonary hypertension
- PIOPED
Prospective Investigation On Pulmonary Embolism Diagnosis
- PVR
pulmonary vascular resistance
- RIETE
Registro Informatizado de la Enfermedad Thromboembolica venosa
- RR
relative risk
- rtPA
recombinant tissue plasminogen activator
- RV
right ventricle/ventricular
- SPECT
single photon emission computed tomography
- sPESI
simplified pulmonary embolism severity index
- TAPSE
tricuspid annulus plane systolic excursion
- Tc
technetium
- TOE
transoesophageal echocardiography
- TTR
time in therapeutic range
- TV
tricuspid valve
- UFH
unfractionated heparin
- V/Q scan
ventilation–perfusion scintigraphy
- VKA
vitamin K antagonist(s)
- VTE
venous thromboembolism
1. Preamble
Guidelines summarize and evaluate all available evidence at the time of the writing process, on a particular issue with the aim of assisting health professionals in selecting the best management strategies for an individual patient, with a given condition, taking into account the impact on outcome, as well as the risk-benefit-ratio of particular diagnostic or therapeutic means. Guidelines and recommendations should help the health professionals to make decisions in their daily practice. However, the final decisions concerning an individual patient must be made by the responsible health professional(s) in consultation with the patient and caregiver as appropriate.
A great number of Guidelines have been issued in recent years by the European Society of Cardiology (ESC) as well as by other societies and organisations. Because of the impact on clinical practice, quality criteria for the development of guidelines have been established in order to make all decisions transparent to the user. The recommendations for formulating and issuing ESC Guidelines can be found on the ESC Web Site (http://www.escardio.org/guidelines-surveys/esc-guidelines/about/Pages/rules-writing.aspx). ESC Guidelines represent the official position of the ESC on a given topic and are regularly updated.
Members of this Task Force were selected by the ESC to represent professionals involved with the medical care of patients with this pathology. Selected experts in the field undertook a comprehensive review of the published evidence for management (including diagnosis, treatment, prevention and rehabilitation) of a given condition according to ESC Committee for Practice Guidelines (CPG) policy. A critical evaluation of diagnostic and therapeutic procedures was performed including assessment of the risk-benefit-ratio. Estimates of expected health outcomes for larger populations were included, where data exist. The level of evidence and the strength of recommendation of particular management options were weighed and graded according to predefined scales, as outlined in Tables 1 and 2.
The experts of the writing and reviewing panels filled in declarations of interest forms which might be perceived as real or potential sources of conflicts of interest. These forms were compiled into one file and can be found on the ESC Web Site (http://www.escardio.org/guidelines). Any changes in declarations of interest that arise during the writing period must be notified to the ESC and updated. The Task Force received its entire financial support from the ESC without any involvement from healthcare industry.
The ESC CPG supervises and coordinates the preparation of new Guidelines produced by Task Forces, expert groups or consensus panels. The Committee is also responsible for the endorsement process of these Guidelines. The ESC Guidelines undergo extensive review by the CPG and external experts. After appropriate revisions it is approved by all the experts involved in the Task Force. The finalized document is approved by the CPG for publication in the European Heart Journal. It was developed after careful consideration of the scientific and medical knowledge and the evidence available at the time of their dating.
The task of developing ESC Guidelines covers not only the integration of the most recent research, but also the creation of educational tools and implementation programmes for the recommendations. To implement the guidelines, condensed pocket guidelines versions, summary slides, booklets with essential messages, summary cards for non-specialists, electronic version for digital applications (smartphones etc) are produced. These versions are abridged and, thus, if needed, one should always refer to the full text version which is freely available on the ESC Website. The National Societies of the ESC are encouraged to endorse, translate and implement the ESC Guidelines. Implementation programmes are needed because it has been shown that the outcome of disease may be favourably influenced by the thorough application of clinical recommendations.
Surveys and registries are needed to verify that real-life daily practice is in keeping with what is recommended in the guidelines, thus completing the loop between clinical research, writing of guidelines, disseminating them and implementing them into clinical practice.
Health professionals are encouraged to take the ESC Guidelines fully into account when exercising their clinical judgment as well as in the determination and the implementation of preventive, diagnostic or therapeutic medical strategies. However, the ESC Guidelines do not override in any way whatsoever the individual responsibility of health professionals to make appropriate and accurate decisions in consideration of each patient's health condition and in consultation with that patient and the patient's caregiver where appropriate and/or necessary. It is also the health professional's responsibility to verify the rules and regulations applicable to drugs and devices at the time of prescription.
2. Introduction
This document follows the two previous ESC Guidelines focussing on clinical management of pulmonary embolism, published in 2000 and 2008. Many recommendations have retained or reinforced their validity; however, new data has extended or modified our knowledge in respect of optimal diagnosis, assessment and treatment of patients with PE. The most clinically relevant new aspects of this 2014 version as compared with its previous version published in 2008 relate to: These new aspects have been integrated into previous knowledge to suggest optimal and—whenever possible—objectively validated management strategies for patients with suspected or confirmed pulmonary embolism.
Recently identified predisposing factors for venous thromboembolism
Simplification of clinical prediction rules
Age-adjusted D-dimer cut-offs
Sub-segmental pulmonary embolism
Incidental, clinically unsuspected pulmonary embolism
Advanced risk stratification of intermediate-risk pulmonary embolism
Initiation of treatment with vitamin K antagonists
Treatment and secondary prophylaxis of venous thromboembolism with the new direct oral anticoagulants
Efficacy and safety of reperfusion treatment for patients at intermediate risk
Early discharge and home (outpatient) treatment of pulmonary embolism
Current diagnosis and treatment of chronic thromboembolic pulmonary hypertension
Formal recommendations for the management of pulmonary embolism in pregnancy and of pulmonary embolism in patients with cancer.
In order to limit the length of the printed text, additional information, tables, figures and references are available as web addenda at the ESC website (www.escardio.org).
2.1 Epidemiology
Venous thromboembolism (VTE) encompasses deep vein thrombosis (DVT) and pulmonary embolism (PE). It is the third most frequent cardiovascular disease with an overall annual incidence of 100–200 per 100 000 inhabitants.1,2 VTE may be lethal in the acute phase or lead to chronic disease and disability,3–6 but it is also often preventable.
Acute PE is the most serious clinical presentation of VTE. Since PE is, in most cases, the consequence of DVT, most of the existing data on its epidemiology, risk factors, and natural history are derived from studies that have examined VTE as a whole.
The epidemiology of PE is difficult to determine because it may remain asymptomatic, or its diagnosis may be an incidental finding;2 in some cases, the first presentation of PE may be sudden death.7,8 Overall, PE is a major cause of mortality, morbidity, and hospitalization in Europe. As estimated on the basis of an epidemiological model, over 317 000 deaths were related to VTE in six countries of the European Union (with a total population of 454.4 million) in 2004.2 Of these cases, 34% presented with sudden fatal PE and 59% were deaths resulting from PE that remained undiagnosed during life; only 7% of the patients who died early were correctly diagnosed with PE before death. Since patients older than 40 years are at increased risk compared with younger patients and the risk approximately doubles with each subsequent decade, an ever-larger number of patients are expected to be diagnosed with (and perhaps die of) PE in the future.9
In children, studies reported an annual incidence of VTE between 53 and 57 per 100 000 among hospitalized patients,10,11 and between 1.4 and 4.9 per 100 000 in the community at large.12,13
2.2 Predisposing factors
A list of predisposing (risk) factors for VTE is shown in Web Addenda Table I. There is an extensive collection of predisposing environmental and genetic factors. VTE is considered to be a consequence of the interaction between patient-related—usually permanent—risk factors and setting-related—usually temporary—risk factors. VTE is considered to be ‘provoked’ in the presence of a temporary or reversible risk factor (such as surgery, trauma, immobilization, pregnancy, oral contraceptive use or hormone replacement therapy) within the last 6 weeks to 3 months before diagnosis,14 and ‘unprovoked’ in the absence thereof. PE may also occur in the absence of any known risk factor. The presence of persistent—as opposed to major, temporary—risk factors may affect the decision on the duration of anticoagulation therapy after a first episode of PE.
Major trauma, surgery, lower limb fractures and joint replacements, and spinal cord injury, are strong provoking factors for VTE.9,15 Cancer is a well-recognized predisposing factor for VTE. The risk of VTE varies with different types of cancer;16,17 haematological malignancies, lung cancer, gastrointestinal cancer, pancreatic cancer and brain cancer carry the highest risk.18,19 Moreover, cancer is a strong risk factor for all-cause mortality following an episode of VTE.20
In fertile women, oral contraception is the most frequent predisposing factor for VTE.21,22 When occurring during pregnancy, VTE is a major cause of maternal mortality.23 The risk is highest in the third trimester of pregnancy and over the 6 weeks of the postpartum period, being up to 60 times higher 3 months after delivery, compared with the risk in non-pregnant women.23In vitro fertilization further increases the risk of pregnancy-associated VTE. In a cross-sectional study derived from a Swedish registry, the overall risk of PE (compared with the risk of age-matched women whose first child was born without in vitro fertilization) was particularly increased during the first trimester of pregnancy [hazard ratio (HR) 6.97; 95% confidence interval (CI) 2.21–21.96]. The absolute number of women who suffered PE was low in both groups (3.0 vs. 0.4 cases per 10 000 pregnancies during the first trimester, and 8.1 vs. 6.0 per 10 000 pregnancies overall).24 In post-menopausal women who receive hormone replacement therapy, the risk of VTE varies widely depending on the formulation used.25
Infection has been found to be a common trigger for hospitalization for VTE.15,26,27 Blood transfusion and erythropoiesis-stimulating agents are also associated with an increased risk of VTE.15,28
In children, PE is usually associated with DVT and is rarely unprovoked. Serious chronic medical conditions and central venous lines are considered to be likely triggers of PE.29
VTE may be viewed as part of the cardiovascular disease continuum and common risk factors—such as cigarette smoking, obesity, hypercholesterolaemia, hypertension and diabetes mellitus30–33—are shared with arterial disease, notably atherosclerosis.34–37 However, at least in part, this may be an indirect association, mediated by the effects of coronary artery disease and, in the case of smoking, cancer.38,39 Myocardial infarction and heart failure increase the risk of PE;40,41conversely, patients with VTE have an increased risk of subsequent myocardial infarction and stroke.42
2.3 Natural history
The first studies on the natural history of VTE were carried out in the setting of orthopaedic surgery during the 1960s.43 Evidence collected since this initial report has shown that DVT develops less frequently in non-orthopaedic surgery. The risk of VTE is highest during the first two post-operative weeks but remains elevated for two to three months. Antithrombotic prophylaxis significantly reduces the risk of perioperative VTE. The incidence of VTE is reduced with increasing duration of thromboprophylaxis after major orthopaedic surgery and (to a lesser extent) cancer surgery: this association has not been shown for general surgery.44,45 The majority of patients with symptomatic DVT have proximal clots, complicated by PE in 40–50% of cases, often without clinical manifestations.44,45
Registries and hospital discharge datasets of unselected patients with PE or VTE yielded 30-day all-cause mortality rates between 9% and 11%, and three-month mortality ranging between 8.6% and 17%.46–48 Following the acute PE episode, resolution of pulmonary thrombi, as evidenced by lung perfusion defects, is frequently incomplete. In one study, lung perfusion scintigraphy demonstrated abnormalities in 35% of patients a year after acute PE, although the degree of pulmonary vascular obstruction was <15% in 90% of the cases.49 Two relatively recent cohort studies covering 173 and 254 patients yielded incidences approaching 30%.50,51 The incidence of confirmed chronic thromboembolic pulmonary hypertension (CTEPH) after unprovoked PE is currently estimated at approximately 1.5% (with a wide range reported by mostly small-cohort studies), with most cases appearing within 24 months of the index event.52,53
The risk of recurrence of VTE has been reviewed in detail.54–56 Based on historical data, the cumulative proportion of patients with early recurrence of VTE (on anticoagulant treatment) amounts to 2.0% at 2 weeks, 6.4% at 3 months and 8% at 6 months; more recent, randomized anticoagulation trials (discussed in the section on acute phase treatment) indicate that recurrence rates may have dropped considerably recently. The rate of recurrence is highest during the first two weeks and declines thereafter. During the early period, active cancer and failure to rapidly achieve therapeutic levels of anticoagulation appear to independently predict an increased risk of recurrence.56,57
The cumulative proportion of patients with late recurrence of VTE (after six months, and in most cases after discontinuation of anticoagulation) has been reported to reach 13% at 1 year, 23% at 5 years, and 30% at 10 years.56 Overall, the frequency of recurrence does not appear to depend on the clinical presentation (DVT or PE) of the first event, but recurrent VTE is likely to occur in the same clinical form as the index episode (i.e. if VTE recurs after PE, it will most likely be PE again). Recurrence is more frequent after multiple VTE episodes as opposed to a single event, and after unprovoked VTE as opposed to the presence of temporary risk factors, particularly surgery.58 It is also more frequent in women who continue hormone intake after a VTE episode, and in patients who have suffered PE or proximal vein thrombosis compared to distal (calf) vein thrombosis. On the other hand, factors for which an independent association with late recurrence have not been definitely established include age, male sex,59,60 a family history of VTE, and an increased body mass index.54,56 Elevated D-dimer levels, either during or after discontinuation of anticoagulation, indicate an increased risk of recurrence;61–63 on the other hand, single thrombophilic defects have a low predictive value and anticoagulation management based on thrombophilia testing has not been found to reduce VTE recurrence.64,65
2.4 Pathophysiology
Acute PE interferes with both the circulation and gas exchange. Right ventricular (RV) failure due to pressure overload is considered the primary cause of death in severe PE.
Pulmonary artery pressure increases only if more than 30–50% of the total cross-sectional area of the pulmonary arterial bed is occluded by thromboemboli.66 PE-induced vasoconstriction, mediated by the release of thromboxane A2 and serotonin, contributes to the initial increase in pulmonary vascular resistance after PE,67 an effect that can be reversed by vasodilators.68,69 Anatomical obstruction and vasoconstriction lead to an increase in pulmonary vascular resistance and a proportional decrease in arterial compliance.70
The abrupt increase in pulmonary vascular resistance results in RV dilation, which alters the contractile properties of the RV myocardium via the Frank-Starling mechanism. The increase in RV pressure and volume leads to an increase in wall tension and myocyte stretch. RV contraction time is prolonged, while neurohumoral activation leads to inotropic and chronotropic stimulation. Together with systemic vasoconstriction, these compensatory mechanisms increase pulmonary artery pressure, improving flow through the obstructed pulmonary vascular bed, and thus temporarily stabilize systemic blood pressure (BP).71 The extent of immediate adaptation is limited, since a non-preconditioned, thin-walled right ventricle (RV) is unable to generate a mean pulmonary artery pressure above 40 mm Hg.
The prolongation of RV contraction time into early diastole in the left ventricle leads to leftward bowing of the interventricular septum.72 The desynchronization of the ventricles may be exacerbated by the development of right bundle-branch block. As a result, left ventricular (LV) filling is impeded in early diastole, and this may lead to a reduction of the cardiac output and contribute to systemic hypotension and haemodynamic instability.73
As described above, excessive neurohumoral activation in PE can be the result both of abnormal RV wall tension and of circulatory shock. The finding of massive infiltrates in the RV myocardium of patients who died within 48 hours of acute PE may be explained by high levels of epinephrine released as a result of the PE-induced ‘myocarditis’.74 This inflammatory response might explain the secondary haemodynamic destabilization which sometimes occurs 24–48 hours after acute PE, although early recurrence of PE may be an alternative explanation in some of these cases.75
Finally, the association between elevated circulating levels of biomarkers of myocardial injury and an adverse early outcome indicates that RV ischaemia is of pathophysiological significance in the acute phase of PE.76–78 Although RV infarction is uncommon after PE, it is likely that the imbalance between oxygen supply and demand can result in damage to cardiomyocytes and further reduce contractile forces.
The detrimental effects of acute PE on the RV myocardium and the circulation are summarized in Figure 1.
Key factors contributing to haemodynamic collapse in acute pulmonary embolism
Respiratory failure in PE is predominantly a consequence of haemodynamic disturbances.79 Low cardiac output results in desaturation of the mixed venous blood. In addition, zones of reduced flow in obstructed vessels, combined with zones of overflow in the capillary bed served by non-obstructed vessels, result in ventilation–perfusion mismatch, which contributes to hypoxaemia. In about one-third of patients, right-to-left shunting through a patent foramen ovale can be detected by echocardiography: this is caused by an inverted pressure gradient between the right atrium and left atrium and may lead to severe hypoxaemia and an increased risk of paradoxical embolization and stroke.80 Finally, even if they do not affect haemodynamics, small distal emboli may create areas of alveolar haemorrhage resulting in haemoptysis, pleuritis, and pleural effusion, which is usually mild. This clinical presentation is known as ‘pulmonary infarction’. Its effect on gas exchange is normally mild, except in patients with pre-existing cardiorespiratory disease.
2.5 Clinical classification of pulmonary embolism severity
The clinical classification of the severity of an episode of acute PE is based on the estimated PE-related early mortality risk defined by in-hospital or 30-day mortality (Figure 2). This stratification, which has important implications both for the diagnostic and therapeutic strategies proposed in these guidelines, is based on the patient's clinical status at presentation, with high-risk PE being suspected or confirmed in the presence of shock or persistent arterial hypotension and not high-risk PE in their absence.
3. Diagnosis
Throughout these Guidelines and for the purpose of clinical management, ‘confirmed PE’ is defined as a probability of PE high enough to indicate the need for PE-specific treatment, and ‘excluded PE’ as a probability of PE low enough to justify withholding PE-specific treatment with an acceptably low risk.
3.1 Clinical presentation
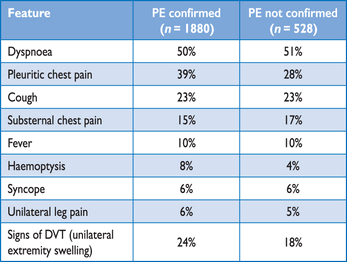
PE may escape prompt diagnosis since the clinical signs and symptoms are non-specific (Table 3). When the clinical presentation raises the suspicion of PE in an individual patient, it should prompt further objective testing. In most patients, PE is suspected on the basis of dyspnoea, chest pain, pre-syncope or syncope, and/or haemoptysis.81–83 Arterial hypotension and shock are rare but important clinical presentations, since they indicate central PE and/or a severely reduced haemodynamic reserve. Syncope is infrequent, but may occur regardless of the presence of haemodynamic instability.84 Finally, PE may be completely asymptomatic and be discovered incidentally during diagnostic work-up for another disease or at autopsy.
Clinical characteristics of patients with suspected PE in the emergency department (adapted from Pollack et al. (2011)).82
 |
 |
DVT = deep vein thrombosis.
Clinical characteristics of patients with suspected PE in the emergency department (adapted from Pollack et al. (2011)).82
 |
 |
DVT = deep vein thrombosis.
Chest pain is a frequent symptom of PE and is usually caused by pleural irritation due to distal emboli causing pulmonary infarction.85 In central PE, chest pain may have a typical angina character, possibly reflecting RV ischaemia and requiring differential diagnosis with acute coronary syndrome (ACS) or aortic dissection. Dyspnoea may be acute and severe in central PE; in small peripheral PE, it is often mild and may be transient. In patients with pre-existing heart failure or pulmonary disease, worsening dyspnoea may be the only symptom indicative of PE.
Knowledge of the predisposing factors for VTE is important in determining the likelihood of PE, which increases with the number of predisposing factors present; however, in as many as 30% of the patients with PE, no provoking factors can be detected.86 In blood gas analysis, hypoxaemia is considered a typical finding in acute PE, but up to 40% of the patients have normal arterial oxygen saturation and 20% a normal alveolar-arterial oxygen gradient.87,88 Hypocapnia is also often present. The chest X-ray is frequently abnormal and, although its findings are usually non-specific in PE, it is useful for excluding other causes of dyspnoea or chest pain.89 Electrocardiographic changes indicative of RV strain, such as inversion of T waves in leads V1–V4, a QR pattern in V1, S1Q3T3 pattern, and incomplete or complete right bundle-branch block, may be helpful. These electrocardiographic changes are usually found in more severe cases of PE;90 in milder cases, the only anomaly may be sinus tachycardia, present in 40% of patients. Finally, atrial arrhythmias, most frequently atrial fibrillation, may be associated with acute PE.
3.2 Assessment of clinical probability
Despite the limited sensitivity and specificity of individual symptoms, signs, and common tests, the combination of findings evaluated by clinical judgement or by the use of prediction rules allows to classify patients with suspected PE into distinct categories of clinical or pre-test probability that correspond to an increasing actual prevalence of confirmed PE. As the post-test (e.g. after computed tomography) probability of PE depends not only on the characteristics of the diagnostic test itself but also on pre-test probability, this has become a key step in all diagnostic algorithms for PE.
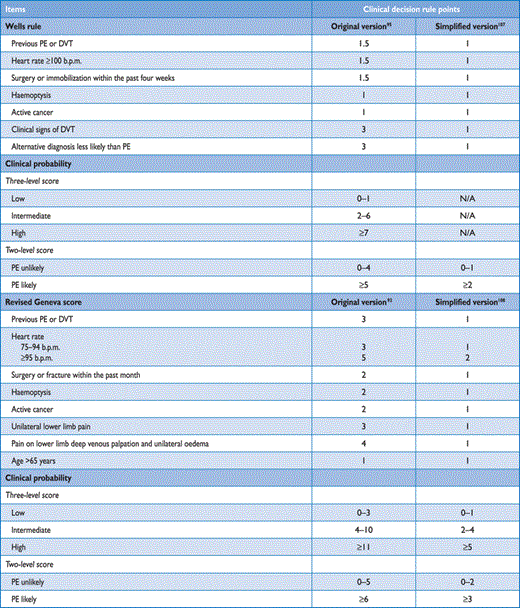
The value of clinical judgement has been confirmed in several large series,91–93 including the Prospective Investigation On Pulmonary Embolism Diagnosis (PIOPED).94 Note that clinical judgement usually includes commonplace tests such as chest X-ray and electrocardiogram for differential diagnosis. However, clinical judgement lacks standardization; therefore, several explicit clinical prediction rules have been developed. Of these, the most frequently used prediction rule is the one offered by Wells et al. (Table 4).95 This rule has been validated extensively using both a three-category scheme (low, moderate, or high clinical probability of PE) and a two-category scheme (PE likely or unlikely).96–100 It is simple and based on information that is easy to obtain; on the other hand, the weight of one subjective item (‘alternative diagnosis less likely than PE’) may reduce the inter-observer reproducibility of the Wells rule.101–103 The revised Geneva rule is also simple and standardized (Table 4).93 Both have been adequately validated.104–106
Clinical prediction rules for PE
 |
 |
b.p.m.= beats per minute; DVT = deep vein thrombosis; PE = pulmonary embolism.
Clinical prediction rules for PE
 |
 |
b.p.m.= beats per minute; DVT = deep vein thrombosis; PE = pulmonary embolism.
More recently, both the Wells and the revised Geneva rule were simplified in an attempt to increase their adoption into clinical practice (Table 4),107,108 and the simplified versions were externally validated.105,109 Whichever is used, the proportion of patients with confirmed PE can be expected to be around 10% in the low-probability category, 30% in the moderate-probability category, and 65% in the high-clinical probability category when using the three-level classification.104 When the two-level classification is used, the proportion of patients with confirmed PE in the PE-unlikely category is around 12%.104
3.3 D-dimer testing
D-dimer levels are elevated in plasma in the presence of acute thrombosis because of simultaneous activation of coagulation and fibrinolysis. The negative predictive value of D-dimer testing is high and a normal D-dimer level renders acute PE or DVT unlikely. On the other hand, fibrin is also produced in a wide variety of conditions such as cancer, inflammation, bleeding, trauma, surgery and necrosis. Accordingly, the positive predictive value of elevated D-dimer levels is low and D-dimer testing is not useful for confirmation of PE.
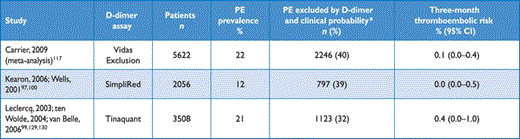
A number of D-dimer assays are available.110,111 The quantitative enzyme-linked immunosorbent assay (ELISA) or ELISA-derived assays have a diagnostic sensitivity of 95% or better and can therefore be used to exclude PE in patients with either a low or a moderate pre-test probability. In the emergency department, a negative ELISA D-dimer, in combination with clinical probability, can exclude the disease without further testing in approximately 30% of patients with suspected PE.100,112,113 Outcome studies have shown that the three-month thromboembolic risk was <1% in patients left untreated on the basis of a negative test result (Table 5);99,112–116 these findings were confirmed by a meta-analysis.117
Diagnostic yield of various D-dimer assays in excluding acute PE according to outcome studies
 |
 |
CI = confidence interval; PE = pulmonary embolism.
aLow or intermediate clinical probability, or PE unlikely, depending on the studies.
Diagnostic yield of various D-dimer assays in excluding acute PE according to outcome studies
 |
 |
CI = confidence interval; PE = pulmonary embolism.
aLow or intermediate clinical probability, or PE unlikely, depending on the studies.
Quantitative latex-derived assays and a whole-blood agglutination assay have a diagnostic sensitivity <95% and are thus often referred to as moderately sensitive. In outcome studies, those assays proved safe in ruling out PE in PE-unlikely patients as well as in patients with a low clinical probability.99,100,105 Their safety in ruling out PE has not been established in the intermediate clinical probability category. Point-of-care tests have moderate sensitivity, and data from outcome studies in PE are lacking, with the exception of a recent primary care-based study using the Simplify D-dimer assay,118 in which the three-month thromboembolic risk was 1.5% in PE-unlikely patients with a negative D-dimer.
The specificity of D-dimer in suspected PE decreases steadily with age, to almost 10% in patients >80 years.119 Recent evidence suggests using age-adjusted cut-offs to improve the performance of D-dimer testing in the elderly.120,121 In a recent meta-analysis, age-adjusted cut-off values (age x 10 µg/L above 50 years) allowed increasing specificity from 34–46% while retaining a sensitivity above 97%.122 A multicentre, prospective management study evaluated this age-adjusted cut-off in a cohort of 3346 patients. Patients with a normal age-adjusted D-dimer value did not undergo computed tomographic pulmonary angiography and were left untreated and formally followed up for a three-month period. Among the 766 patients who were 75 years or older, 673 had a non-high clinical probability. On the basis of D-dimer, using the age-adjusted cut-off (instead of the ‘standard’ 500 µg/L cut-off) increased the number of patients in whom PE could be excluded from 43 (6.4%; 95% CI 4.8–8.5%) to 200 (29.7%; 95% CI 26.4–33.3%), without any additional false-negative findings.123 D-dimer is also more frequently elevated in patients with cancer,124,125 in hospitalized patients,105,126 and during pregnancy.127,128 Thus, the number of patients in whom D-dimer must be measured to exclude one PE (number needed to test) varies between 3 in the emergency department and ≥10 in the specific situations listed above. The negative predictive value of a (negative) D-dimer test remains high in these situations.
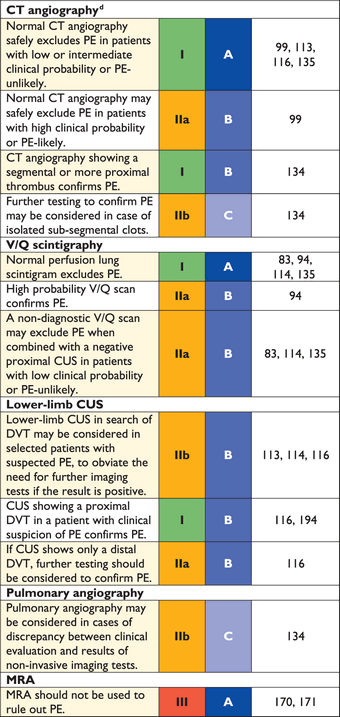
3.4 Computed tomographic pulmonary angiography
Since the introduction of multi-detector computed tomographic (MDCT) angiography with high spatial and temporal resolution and quality of arterial opacification, computed tomographic (CT) angiography has become the method of choice for imaging the pulmonary vasculature in patients with suspected PE. It allows adequate visualization of the pulmonary arteries down to at least the segmental level.131–133 The PIOPED II trial observed a sensitivity of 83% and a specificity of 96% for (mainly four-detector) MDCT.134 PIOPED II also highlighted the influence of clinical probability on the predictive value of MDCT. In patients with a low or intermediate clinical probability of PE as assessed by the Wells rule, a negative CT had a high negative predictive value for PE (96% and 89%, respectively), whereas this was only 60% in those with a high pre-test probability. Conversely, the positive predictive value of a positive CT was high (92–96%) in patients with an intermediate or high clinical probability but much lower (58%) in patients with a low pre-test likelihood of PE. Therefore, clinicians should be particularly cautious in case of discordancy between clinical judgement and the MDCT result.
Four studies provided evidence in favour of computed tomography as a stand-alone imaging test for excluding PE. In a prospective management study covering 756 consecutive patients referred to the emergency department with a clinical suspicion of PE, all patients with either a high clinical probability or a non-high clinical probability and a positive ELISA D-dimer test underwent both lower limb ultrasonography and MDCT.113 The proportion of patients in whom—despite a negative MDCT—a proximal DVT was found on ultrasound was only 0.9% (95% CI 0.3–2.7).113 In another study,99 all patients classified as PE-likely by the dichotomized Wells rule, or those with a positive D-dimer test, underwent a chest MDCT. The three-month thromboembolic risk in the patients left untreated because of a negative CT was low (1.1%; 95% CI 0.6–1.9).99 Two randomized, controlled trials reached similar conclusions. In a Canadian trial comparing V/Q scan and CT (mostly MDCT), only seven of the 531 patients (1.3%) with a negative CT had a DVT, and one had a thromboembolic event during follow-up.135 Hence, the three-month thromboembolic risk would have been 1.5% (95% CI 0.8–2.9) if only CT had been used.135 A European study compared two diagnostic strategies based on D-dimer and MDCT, one with- and the other without lower limb compression venous ultrasonography (CUS).116 In the D-dimer–CT arm, the three-month thromboembolic risk was 0.3% (95% CI 0.1–1.2) among the 627 patients left untreated, based on a negative D-dimer or MDCT.
Taken together, these data suggest that a negative MDCT is an adequate criterion for excluding PE in patients with a non-high clinical probability of PE. Whether patients with a negative CT and a high clinical probability should be further investigated is controversial. MDCT showing PE at the segmental or more proximal level is adequate proof of PE in patients with a non-low clinical probability; however, the positive predictive value of MDCT is lower in patients with a low clinical probability of PE, and further testing may be considered, especially if the clots are limited to segmental or sub-segmental arteries.
The clinical significance of isolated sub-segmental PE on CT angiography is questionable. This finding was present in 4.7% (2.5–7.6%) of patients with PE imaged by single-detector CT angiography and 9.4% (5.5–14.2%) of those submitted to MDCT.136 The positive predictive value is low and inter-observer agreement is poor at this distal level.137 There may be a role for CUS in this situation, to ensure that the patient does not have DVT that would require treatment. In a patient with isolated sub-segmental PE and no proximal DVT, the decision on whether to treat should be made on an individual basis, taking into account the clinical probability and the bleeding risk.
Computed tomographic venography has been advocated as a simple way to diagnose DVT in patients with suspected PE, as it can be combined with chest CT angiography as a single procedure, using only one intravenous injection of contrast dye. In PIOPED II, combining CT venography with CT angiography increased sensitivity for PE from 83% to 90% and had a similar specificity (around 95%);134,138 however, the corresponding increase in negative predictive value was not clinically significant. CT venography adds a significant amount of irradiation, which may be a concern, especially in younger women.139 As CT venography and CUS yielded similar results in patients with signs or symptoms of DVT in PIOPED II,138 ultrasonography should be used instead of CT venography if indicated (see Section 3.10).
The incidental discovery of clinically unsuspected PE on CT is an increasingly frequent problem, arising in 1–2% of all thoracic CT examinations, most often in patients with cancer, but also among those with paroxysmal atrial fibrillation or heart failure and history of atrial fibrillation.140–143 There are no robust data to guide the decision on how to manage unsuspected PE with anticoagulants, but most experts agree that patients with cancer and those with clots at the lobar or more proximal level should be treated with anticoagulants.144
3.5 Lung scintigraphy
Ventilation–perfusion scintigraphy (V/Q scan) is an established diagnostic test for suspected PE. It is safe and few allergic reactions have been described. The test is based on the intravenous injection of technetium (Tc)-99m-labelled macroaggregated albumin particles, which block a small fraction of the pulmonary capillaries and thereby enable scintigraphic assessment of lung perfusion. Perfusion scans are combined with ventilation studies, for which multiple tracers such as xenon-133 gas, Tc-99m-labelled aerosols, or Tc-99m-labelled carbon microparticles (Technegas) can be used. The purpose of the ventilation scan is to increase specificity: in acute PE, ventilation is expected to be normal in hypoperfused segments (mismatch).145,146 According to the International Commission on Radiological Protection (ICRP), the radiation exposure from a lung scan with 100 MBq of Tc-99m macroaggregated albumin particles is 1.1 mSv for an average sized adult, and thus is significantly lower than that of CT angiography (2–6 mSv).147,148
Being a radiation- and contrast medium-sparing procedure, the V/Q scan may preferentially be applied in outpatients with low clinical probability and a normal chest X-ray, in young (particularly female) patients, in pregnancy, in patients with history of contrast medium-induced anaphylaxis and strong allergic history, in severe renal failure, and in patients with myeloma and paraproteinaemia.149
Lung scan results are frequently classified according to the criteria established in the PIOPED study: normal or near-normal, low, intermediate (non-diagnostic), and high probability of PE.94 These criteria have been the subject of debate, following which they were revised.150,151 To facilitate communication with clinicians, a three-tier classification is preferable: normal scan (excluding PE), high-probability scan (considered diagnostic of PE in most patients), and non-diagnostic scan.135,152,153 Prospective clinical outcome studies suggested that it is safe to withhold anticoagulant therapy in patients with a normal perfusion scan. This was recently confirmed by a randomized trial comparing the V/Q scan with CT.135 An analysis from the recent PIOPED II study confirmed the effectiveness of the high-probability V/Q scan for diagnosing PE and of the normal perfusion scan for ruling it out.154 Performing only a perfusion scan is acceptable in patients with a normal chest X-ray; any perfusion defect in this situation will be considered to be a mismatch.155 The high frequency of non-diagnostic intermediate probability scans has been a cause for criticism, because they indicate the necessity for further diagnostic testing. Various strategies to overcome this problem have been proposed, notably the incorporation of clinical probability.91,156,157
Recent studies suggest that data acquisition in the tomographic mode in single photon emission computed tomography (SPECT) imaging, with or without low-dose CT may reduce the frequency of non-diagnostic scans.152,158–161 SPECT imaging may even allow the use of automated detection algorithms for PE.162 Large-scale prospective studies are needed to validate these new approaches.
3.6 Pulmonary angiography
Pulmonary angiography has for decades remained the ‘gold standard' for the diagnosis or exclusion of PE, but is rarely performed now as less-invasive CT angiography offers similar diagnostic accuracy.163 Pulmonary angiography is more often used to guide percutaneous catheter-directed treatment of acute PE. Digital subtraction angiography (DSA) requires less contrast medium than conventional cineangiography and has excellent imaging quality for peripheral pulmonary vessels in patients who can hold their breath; it is less useful for imaging of the main pulmonary arteries, due to cardiac motion artefacts.
The diagnosis of acute PE is based on direct evidence of a thrombus in two projections, either as a filling defect or as amputation of a pulmonary arterial branch.94 Thrombi as small as 1–2 mm within the sub-segmental arteries can be visualized by DSA, but there is substantial inter-observer variability at this level.164,165 Indirect signs of PE, such as slow flow of contrast, regional hypoperfusion, and delayed or diminished pulmonary venous flow, are not validated and hence are not diagnostic. The Miller score may be used in quantifying the extent of luminal obstruction.166
Pulmonary angiography is not free of risk. In a study of 1111 patients, procedure-related mortality was 0.5%, major non-fatal complications occurred in 1%, and minor complications in 5%.167 The majority of deaths occurred in patients with haemodynamic compromise or respiratory failure. The risk of access-related bleeding complications is increased if thrombolysis is attempted in patients with PE diagnosed by pulmonary angiography.168
Haemodynamic measurements should always be recorded during pulmonary angiography for estimation of the severity of PE and because they may suggest alternative cardiopulmonary disorders. In patients with haemodynamic compromise, the amount of contrast agent should be reduced and non-selective injections avoided.169
3.7 Magnetic resonance angiography
Magnetic resonance angiography (MRA) has been evaluated for several years in suspected PE but large-scale studies were published only recently.170,171 Their results show that this technique, although promising, is not yet ready for clinical practice due to its low sensitivity, high proportion of inconclusive MRA scans, and low availability in most emergency settings. The hypothesis—that a negative MRA combined with the absence of proximal DVT on CUS may safely rule out clinically significant PE—is being tested in a multicentre outcome study (ClinicalTrials.gov NCT 02059551).
3.8 Echocardiography
Acute PE may lead to RV pressure overload and dysfunction, which can be detected by echocardiography. Given the peculiar geometry of the RV, there is no individual echocardiographic parameter that provides fast and reliable information on RV size or function. This is why echocardiographic criteria for the diagnosis of PE have differed between studies. Because of the reported negative predictive value of 40–50%, a negative result cannot exclude PE.157,172,173 On the other hand, signs of RV overload or dysfunction may also be found in the absence of acute PE and be due to concomitant cardiac or respiratory disease.174
RV dilation is found in at least 25% of patients with PE, and its detection, either by echocardiography or CT, is useful for risk stratification of the disease. Echocardiographic findings—based either on a disturbed RV ejection pattern (so-called ‘60–60 sign’) or on depressed contractility of the RV free wall compared with the RV apex (‘McConnell sign’)—were reported to retain a high positive predictive value for PE, even in the presence of pre-existing cardiorespiratory disease.175 Additional echocardiographic signs of pressure overload may be required to avoid a false diagnosis of acute PE in patients with RV free wall hypokinesia or akinesia due to RV infarction, which may mimic the McConnell sign.176 Measurement of the tricuspid annulus plane systolic excursion (TAPSE) may also be useful.177 New echocardiographic parameters of RV function, derived from Doppler tissue imaging and wall strain assessment, were reported to be affected by the presence of acute PE, but they are non-specific and may be normal in haemodynamically stable patients, despite the presence of PE.178–181
Echocardiographic examination is not recommended as part of the diagnostic work-up in haemodynamically stable, normotensive patients with suspected (not high-risk) PE.157 This is in contrast to suspected high-risk PE, in which the absence of echocardiographic signs of RV overload or dysfunction practically excludes PE as the cause of haemodynamic instability. In the latter case, echocardiography may be of further help in the differential diagnosis of the cause of shock, by detecting pericardial tamponade, acute valvular dysfunction, severe global or regional LV dysfunction, aortic dissection, or hypovolaemia. Conversely, in a haemodynamically compromised patient with suspected PE, unequivocal signs of RV pressure overload and dysfunction justify emergency reperfusion treatment for PE if immediate CT angiography is not feasible.182
Mobile right heart thrombi are detected by transthoracic or transoesophageal echocardiography (or by CT angiography) in less than 4% of unselected patients with PE,183–185 but their prevalence may reach 18% in the intensive care setting.185 Mobile right heart thrombi essentially confirm the diagnosis of PE and their presence is associated with RV dysfunction and high early mortality.184,186,187 Consequently, transoesophageal echocardiography may be considered when searching for emboli in the main pulmonary arteries in specific clinical situations,188,189 and it can be of diagnostic value in haemodynamically unstable patients due to the high prevalence of bilateral central pulmonary emboli in most of these cases.190
In some patients with suspected acute PE, echocardiography may detect increased RV wall thickness and/or tricuspid insufficiency jet velocity beyond values compatible with acute RV pressure overload. In these cases, chronic pulmonary hypertension, and CTEPH in particular, should be included in the differential diagnosis.
3.9 Compression venous ultrasonography
In the majority of cases, PE originates from DVT in a lower limb. In a study using venography, DVT was found in 70% of patients with proven PE.191 Nowadays, lower limb CUS has largely replaced venography for diagnosing DVT. CUS has a sensitivity >90% and a specificity of approximately 95% for symptomatic DVT.192,193 CUS shows a DVT in 30–50% of patients with PE,116,192,193 and finding a proximal DVT in patients suspected of having PE is considered sufficient to warrant anticoagulant treatment without further testing.194
In the setting of suspected PE, CUS can be limited to a simple four-point examination (groin and popliteal fossa). The only validated diagnostic criterion for DVT is incomplete compressibility of the vein, which indicates the presence of a clot, whereas flow measurements are unreliable. The diagnostic yield of CUS in suspected PE may be increased further by performing complete ultrasonography, which includes the distal veins. Two recent studies assessed the proportion of patients with suspected PE and a positive D-dimer result, in whom a DVT could be detected by complete CUS.195,196 The diagnostic yield of complete CUS was almost twice that of proximal CUS, but a high proportion (26–36%) of patients with distal DVT had no PE on thoracic MDCT. In contrast, a positive proximal CUS result has a high positive predictive value for PE, as confirmed by data from a large prospective outcome study, in which 524 patients underwent both MDCT and CUS. The sensitivity of CUS for the presence of PE on MDCT was 39% and its specificity was 99%.194 The probability of a positive proximal CUS in suspected PE is higher in patients with signs and symptoms related to the leg veins than in asymptomatic patients.192,193
3.10 Diagnostic strategies
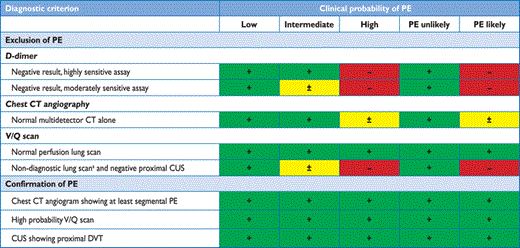
The prevalence of confirmed PE in patients undergoing diagnostic work-up because of suspicion of disease has been rather low (10–35%) in large series.99,100,113,116,197 Hence, the use of diagnostic algorithms is warranted, and various combinations of clinical assessment, plasma D-dimer measurement, and imaging tests have been proposed and validated. These strategies were tested in patients presenting with suspected PE in the emergency ward,99,113,114,116,197 during the hospital stay and more recently in the primary care setting.118,126 Failure to comply with evidence-based diagnostic strategies when withholding anticoagulation was associated with a significant increase in the number of VTE episodes and sudden cardiac death at three-month follow-up.198 The most straightforward diagnostic algorithms for suspected PE—with and without shock or hypotension—are presented in Figures 3 and 4, respectively; however, it is recognized that the diagnostic approach to suspected PE may vary, depending on the availability of—and expertise in—specific tests in various hospitals and clinical settings. Accordingly, Table 6 provides the necessary evidence for alternative evidence-based diagnostic algorithms.
Validated diagnostic criteria (based on non-invasive tests) for diagnosing PE in patients without shock or hypotension according to clinical probability
 |
 |
+/green = valid diagnostic criterion (no further testing required); –/red = invalid criterion (further testing mandatory); ±/yellow = controversial criterion (further testing to be considered).
aLow or intermediate probability lung scan according to the PIOPED classification.
CT = computed tomographic; CUS = proximal lower limb venous ultrasonography; DVT = deep vein thrombosis; PE = pulmonary embolism; PIOPED = Prospective Investigation of Pulmonary Embolism Diagnosis; V/Q scan = ventilation–perfusion scintigram.
Validated diagnostic criteria (based on non-invasive tests) for diagnosing PE in patients without shock or hypotension according to clinical probability
 |
 |
+/green = valid diagnostic criterion (no further testing required); –/red = invalid criterion (further testing mandatory); ±/yellow = controversial criterion (further testing to be considered).
aLow or intermediate probability lung scan according to the PIOPED classification.
CT = computed tomographic; CUS = proximal lower limb venous ultrasonography; DVT = deep vein thrombosis; PE = pulmonary embolism; PIOPED = Prospective Investigation of Pulmonary Embolism Diagnosis; V/Q scan = ventilation–perfusion scintigram.
Proposed diagnostic algorithm for patients with suspected high-risk PE, i.e. presenting with shock or hypotension.
Proposed diagnostic algorithm for patients with suspected not high-risk pulmonary embolism.
The diagnostic strategy for suspected acute PE in pregnancy is discussed in Section 8.1.
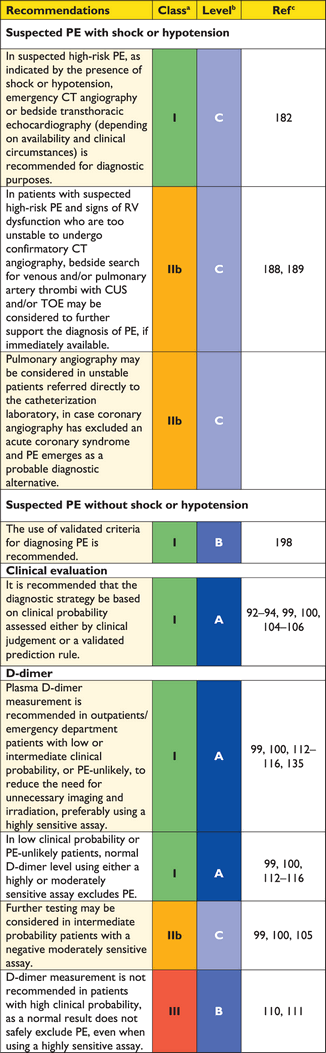
3.10.1 Suspected pulmonary embolism with shock or hypotension
The proposed strategy is shown in Figure 3. Suspected high-risk PE is an immediately life-threatening situation, and patients presenting with shock or hypotension present a distinct clinical problem. The clinical probability is usually high, and the differential diagnosis includes acute valvular dysfunction, tamponade, acute coronary syndrome (ACS), and aortic dissection. The most useful initial test in this situation is bedside transthoracic echocardiography, which will yield evidence of acute pulmonary hypertension and RV dysfunction if acute PE is the cause of the patient's haemodynamic decompensation. In a highly unstable patient, echocardiographic evidence of RV dysfunction is sufficient to prompt immediate reperfusion without further testing. This decision may be strengthened by the (rare) visualization of right heart thrombi.184,199,200 Ancillary bedside imaging tests include transoesophageal echocardiography which, if available, may allow direct visualization of thrombi in the pulmonary artery and its main branches,188,190,201 and bedside CUS, which can detect proximal DVT. As soon as the patient can be stabilized by supportive treatment, final confirmation of the diagnosis by CT angiography should be sought.
For unstable patients admitted directly to the catheterization laboratory with suspected ACS, pulmonary angiography may be considered as a diagnostic procedure after the ACS has been excluded, provided that PE is a probable diagnostic alternative and particularly if percutaneous catheter-directed treatment is a therapeutic option.
3.10.2 Suspected pulmonary embolism without shock or hypotension
Strategy based on computed tomographic angiography (Figure 4)
Computed tomographic angiography has become the main thoracic imaging test for investigating suspected PE but, since most patients with suspected PE do not have the disease, CT should not be the first-line test.
In patients admitted to the emergency department, plasma D-dimer measurement, combined with clinical probability assessment, is the logical first step and allows PE to be ruled out in around 30% of patients, with a three-month thromboembolic risk in patients left untreated of <1%. D-dimer should not be measured in patients with a high clinical probability, owing to a low negative predictive value in this population.202 It is also less useful in hospitalized patients because the number needed to test to obtain a clinically relevant negative result is high.
In most centres, MDCT angiography is the second-line test in patients with an elevated D-dimer level and the first-line test in patients with a high clinical probability. CT angiography is considered to be diagnostic of PE when it shows a clot at least at the segmental level of the pulmonary arterial tree. False-negative results of MDCT have been reported in patients with a high clinical probability of PE;134 however, this situation is infrequent, and the three-month thromboembolic risk was low in these cases.99 Therefore, both the necessity of performing further tests and the nature of these tests in such patients remain controversial.
Value of lower limb compression ultrasonography
Under certain circumstances, CUS can still be useful in the diagnostic work-up of suspected PE. CUS shows a DVT in 30–50% of patients with PE,116,192,193 and finding proximal DVT in a patient suspected of PE is sufficient to warrant anticoagulant treatment without further testing.194 Hence, performing CUS before CT may be an option in patients with relative contraindications for CT such as in renal failure, allergy to contrast dye, or pregnancy.195,196
Value of ventilation–perfusion scintigraphy
In centres in which V/Q scintigraphy is readily available, it remains a valid option for patients with an elevated D-dimer and a contraindication to CT. Also, V/Q scintigraphy may be preferred over CT to avoid unnecessary radiation, particularly in younger and female patients in whom thoracic CT may raise the lifetime risk of breast cancer.139 V/Q lung scintigraphy is diagnostic (with either normal or high-probability findings) in approximately 30–50% of emergency ward patients with suspected PE.83,94,135,203 The proportion of diagnostic V/Q scans is higher in patients with a normal chest X-ray, and this supports the recommendation to use V/Q scan as the first-line imaging test for PE in younger patients.204
The number of patients with inconclusive findings may also be reduced by taking into account clinical probability.94 Thus, patients with a non-diagnostic lung scan and low clinical probability of PE have a low prevalence of confirmed PE.94,157,203 The negative predictive value of this combination is further increased by the absence of a DVT on lower-limb CUS. If a high-probability lung scan is obtained from a patient with low clinical probability of PE, confirmation by other tests may be considered on a case-by-case basis.
3.11 Areas of uncertainty
Despite considerable progress in the diagnosis of PE, several areas of uncertainty persist. The diagnostic value and clinical significance of sub-segmental defects on MDCT are still under debate.136,137 A recent retrospective analysis of two patient cohorts with suspected PE showed similar outcomes (in terms of three-month recurrence and mortality rates) between patients with sub-segmental and more proximal PE; outcomes were largely determined by comorbidities.205 The definition of sub-segmental PE has yet to be standardized and a single sub-segmental defect probably does not have the same clinical relevance as multiple, sub-segmental thrombi.
There is also growing evidence suggesting over-diagnosis of PE.206 A randomized comparison showed that, although CT detected PE more frequently than V/Q scanning, three-month outcomes were similar, regardless of the diagnostic method used.135 Data from the United States show an 80% rise in the apparent incidence of PE after the introduction of CT, without a significant impact on mortality.207,208
Some experts believe that patients with incidental (unsuspected) PE on CT should be treated,144 especially if they have cancer and a proximal clot, but solid evidence in support of this recommendation is lacking. The value and cost-effectiveness of CUS in suspected PE should be further clarified.
Finally, ‘triple rule-out’ (for coronary artery disease, PE and aortic dissection) CT angiography for patients presenting with non-traumatic chest pain appears to be accurate for the detection of coronary artery disease.209 However, the benefits vs. risks (including increased radiation and contrast exposure) of such a diagnostic approach need thorough evaluation, given the low (<1%) prevalence of PE and aortic dissection in the studies published thus far.
Recommendations for diagnosis
 |
 |
 |
 |
CT = computed tomographic (pulmonary angiography); CUS = compression venous ultrasonography; DVT = deep vein thrombosis; MRA = magnetic resonance angiography; PE = pulmonary embolism; RV = right ventricular; TOE = transoesophageal echocardiography; V/Q = ventilation–perfusion.
aClass of recommendation.
bLevel of evidence.
cReferences.
dRefers to multi-detector CT.
4. Prognostic assessment
4.1 Clinical parameters
Acute RV dysfunction is a critical determinant of outcome in acute PE. Accordingly, clinical symptoms and signs of acute RV failure such as persistent arterial hypotension and cardiogenic shock indicate a high risk of early death. Further, syncope and tachycardia—as well as routinely available clinical parameters related to pre-existing conditions and comorbidity—are associated with an unfavourable short-term prognosis. For example, in the International Cooperative Pulmonary Embolism Registry (ICOPER), age >70 years, systolic BP <90 mm Hg, respiratory rate >20 breaths/min, cancer, chronic heart failure and chronic obstructive pulmonary disease (COPD), were all identified as prognostic factors.48 In the Registro Informatizado de la Enfermedad Thomboembolica venosa (RIETE) study, immobilization for neurological disease, age >75 years, and cancer were independently associated with an increased risk of death within the first three months after acute VTE.47 The diagnosis of concomitant DVT has also been reported to be an independent predictor of death within the first three months following diagnosis.210
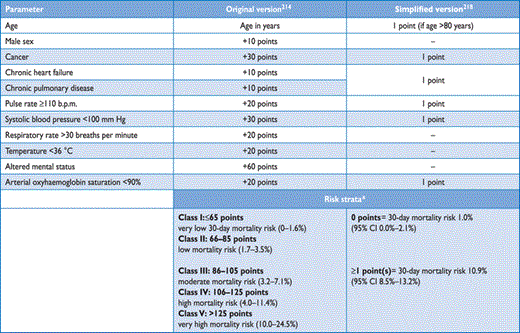
Various prediction rules based on clinical parameters have been shown to be helpful in the prognostic assessment of patients with acute PE. Of those, the pulmonary embolism severity index (PESI; Table 7) is the most extensively validated score to date.211–214 In one study,215 the PESI performed better than the older Geneva prognostic score216 for identification of patients with an adverse 30-day outcome. The principal strength of the PESI lies in the reliable identification of patients at low risk for 30-day mortality (PESI Class I and II). One randomized trial employed a low PESI as the inclusion criterion for home treatment of acute PE.217
Original and simplified PESI
 |
 |
b.p.m. = beats per minute; PESI = Pulmonary embolism severity index.
abased on the sum of points.
Original and simplified PESI
 |
 |
b.p.m. = beats per minute; PESI = Pulmonary embolism severity index.
abased on the sum of points.
Owing to the complexity of the original PESI, which includes 11 differently weighted variables, a simplified version known as sPESI (Table 7) has been developed and validated.218,219 In patients with PE, the sPESI was reported to quantify their 30-day prognosis better than the shock index (defined as heart rate divided by systolic BP),220 and a simplified PESI of 0 was at least as accurate for identification of low-risk patients as the imaging parameters and laboratory biomarkers proposed by the previous ESC Guidelines.221 Combination of the sPESI with troponin testing provided additional prognostic information,222 especially for identification of low-risk patients.76
4.2 Imaging of the right ventricle by echocardiography or computed tomographic angiography
Echocardiographic findings indicating RV dysfunction have been reported in ≥25% of patients with PE.223 They have been identified as independent predictors of an adverse outcome,224 but are heterogeneous and have proven difficult to standardize.225 Still, in haemodynamically stable, normotensive patients with PE, echocardiographic assessment of the morphology and function of the RV may help in prognostic stratification.
As already mentioned in the previous section on the diagnosis of PE, echocardiographic findings used to risk stratify patients with PE include RV dilation, an increased RV–LV diameter ratio, hypokinesia of the free RV wall, increased velocity of the jet of tricuspid regurgitation, decreased tricuspid annulus plane systolic excursion, or combinations of the above. Meta-analyses have shown that RV dysfunction detected by echocardiography is associated with an elevated risk of short-term mortality in patients without haemodynamic instability, but its overall positive predictive value is low (Table 8).226,227 In addition to RV dysfunction, echocardiography can also identify right-to-left shunt through a patent foramen ovale and the presence of right heart thrombi, both of which are associated with increased mortality in patients with acute PE.80,184
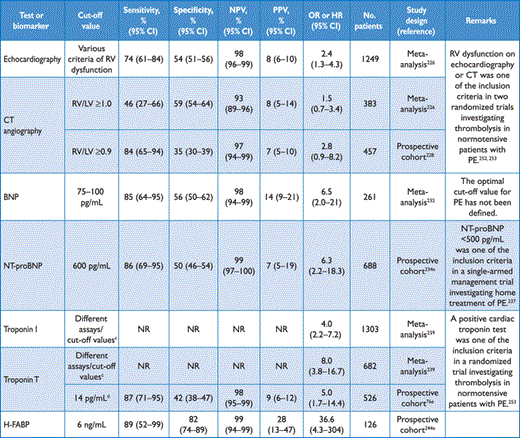
Imaging and laboratory testsa for prediction of earlyb mortality in acute PE
 |
 |
BNP = brain natriuretic peptide; CT = computed tomographic; H-FABP = heart-type fatty acid-binding protein; HR = hazard ratio; LV = left ventricular; NPV = negative predictive value; NR = not reported in the reference cited; NT-proBNP = N-terminal pro-brain natriuretic peptide; OR = odds ratio; PE = pulmonary embolism; PPV = positive predictive value; RV = right ventricular.
aThe Table shows the results of meta-analyses or, in the absence thereof, of the largest prospective cohort studies.
bIn most studies, ‘early’ refers to the in-hospital period or the first 30 days after the index event.
cIn the studies included in this meta-analysis, cut-off values for the cardiac troponin tests used corresponded to the 99thpercentile of healthy subjects with a coefficient variation of <10%.
dHigh-sensitivity assay.
eThese studies included only normotensive patients and used a combined outcome (all-cause death or major cardiovascular complications).
Imaging and laboratory testsa for prediction of earlyb mortality in acute PE
 |
 |
BNP = brain natriuretic peptide; CT = computed tomographic; H-FABP = heart-type fatty acid-binding protein; HR = hazard ratio; LV = left ventricular; NPV = negative predictive value; NR = not reported in the reference cited; NT-proBNP = N-terminal pro-brain natriuretic peptide; OR = odds ratio; PE = pulmonary embolism; PPV = positive predictive value; RV = right ventricular.
aThe Table shows the results of meta-analyses or, in the absence thereof, of the largest prospective cohort studies.
bIn most studies, ‘early’ refers to the in-hospital period or the first 30 days after the index event.
cIn the studies included in this meta-analysis, cut-off values for the cardiac troponin tests used corresponded to the 99thpercentile of healthy subjects with a coefficient variation of <10%.
dHigh-sensitivity assay.
eThese studies included only normotensive patients and used a combined outcome (all-cause death or major cardiovascular complications).
Four-chamber views of the heart by CT angiography may detect RV enlargement (end-diastolic diameter, compared with that of the left ventricle) as an indicator of RV dysfunction. Following a number of early retrospective studies,227 the prognostic value of an enlarged RV on CT angiography was confirmed by a prospective multicentre cohort study of 457 patients (Table 8).228 In-hospital death or clinical deterioration occurred in 44 patients with- and in 8 patients without RV dysfunction on CT (14.5% vs. 5.2%; P < 0.004). Right ventricular dysfunction was an independent predictor for an adverse in-hospital outcome, both in the overall population (HR 3.5; 95% CI 1.6–7.7; P = 0.002) and in haemodynamically stable patients (HR 3.8; 95% CI 1.3–10.9; P = 0.007). Additional recent publications have confirmed these findings.229,230
4.3 Laboratory tests and biomarkers
4.3.1 Markers of right ventricular dysfunction
Right ventricular pressure overload is associated with increased myocardial stretch, which leads to the release of brain natriuretic peptide (BNP) or N-terminal (NT)-proBNP. The plasma levels of natriuretic peptides reflect the severity of haemodynamic compromise and (presumably) RV dysfunction in acute PE.231 A meta-analysis found that 51% of 1132 unselected patients with acute PE had elevated BNP or NT-proBNP concentrations on admission. These patients had a 10% risk of early death (95% CI 8.0–13) and a 23% (95% CI 20–26) risk of an adverse clinical outcome.232
In normotensive patients with PE, the positive predictive value of elevated BNP or NT-proBNP concentrations for early mortality is low.233 In a prospective, multicentre cohort study that included 688 patients, NT-proBNP plasma concentrations of 600 pg/mL were identified as the optimal cut-off value for the identification of elevated risk (Table 8).234 On the other hand, low levels of BNP or NT-proBNP can identify patients with a favourable short-term clinical outcome based on their high negative predictive value.226,232,235,236 Haemodynamically stable patients with low NT-proBNP levels may be candidates for early discharge and outpatient treatment.237
4.3.2 Markers of myocardial injury
Transmural RV infarction despite patent coronary arteries has been found at autopsy of patients who died of massive PE.238 Elevated plasma troponin concentrations on admission have been reported in connection with PE and were associated with worse prognosis. A meta-analysis covering a total of 1985 patients showed elevated cardiac troponin I or -T concentrations in approximately 50% of the patients with acute PE (Table 8).239 Elevated troponin concentrations were associated with high mortality both in unselected patients [odds ratio (OR) 9.44; 95% CI 4.14–21.49] and in haemodynamically stable patients [OR 5.90; 95% CI 2.68–12.95], and the results were consistent for troponin I or -T; however, other reports have suggested a limited prognostic value of elevated troponins in normotensive patients.240
The reported positive predictive value of troponin elevation for PE-related early mortality ranges from 12–44%, while the negative predictive value is high, irrespective of the assays and cut-off values used. Recently developed high-sensitivity assays have improved the prognostic performance of this biomarker, particularly with regard to the exclusion of patients with an adverse short-term outcome.241 For example, in a prospective, multicentre cohort of 526 normotensive patients with acute PE, troponin T concentrations <14 pg/mL, measured by a high-sensitivity assay, had a negative predictive value of 98% with regard to a complicated clinical course, which was similar to that of the sPESI.76
Heart-type fatty acid-binding protein (H-FABP), an early marker of myocardial injury, was also found to possess prognostic value in acute PE.242,243 In normotensive patients, circulating H-FABP levels ≥6 ng/mL had a positive predictive value of 28% and a negative predictive value of 99% for an adverse 30-day outcome (Table 8).244 A simple score, based on the presence of tachycardia, syncope, and a positive bedside test for H-FABP, provided prognostic information similar to that of RV dysfunction on echocardiography.245,246
4.3.3 Other (non-cardiac) laboratory biomarkers
Elevated serum creatinine levels and a decreased (calculated) glomerular filtration rate are related to 30-day all-cause mortality in acute PE.247 Elevated neutrophil gelatinase-associated lipocalin (NGAL) and cystatin C, both indicating acute kidney injury, have also been found to be of prognostic value.248 Elevated D-dimer concentrations were associated with increased short-term mortality in some studies,249,250 while levels <1500 ng/mL had a negative predictive value of 99% for excluding three-month all-cause mortality.251
4.4 Combined modalities and scores
In patients with acute PE who appear haemodynamically stable at diagnosis, no individual clinical, imaging, or laboratory finding has been shown to predict risk of an adverse in-hospital outcome that could be considered high enough to justify primary reperfusion. As a result, various combinations of clinical findings with imaging and laboratory tests have been proposed and tested in registries and cohort studies in an attempt to improve risk stratification.222,246,254–259 The clinical relevance of most of these modalities and scores, particularly with regard to the therapeutic implications, remains to be determined; however, the combination of RV dysfunction on the echocardiogram (or CT angiogram) with a positive cardiac troponin test256,260 was used as an inclusion criterion in a recently published randomized thrombolysis trial,261 which enrolled 1006 normotensive patients with acute PE. Patients treated with standard anticoagulation had a 5.6% incidence of death or haemodynamic decompensation within the first 7 days following randomization.253
4.5 Prognostic assessment strategy
For prediction of early (in-hospital or 30-day) outcome in patients with acute PE, both the PE-related risk and the patient's clinical status and comorbidities should be taken into consideration. The definition for level of clinical risk is shown in Table 9. The risk-adjusted therapeutic strategies and algorithms recommended on the basis of this classification are discussed in the following section and summarized in Figure 5.
Classification of patients with acute PE based on early mortality risk
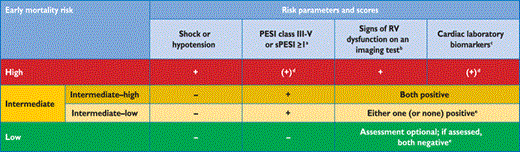 |
 |
PE = pulmonary embolism; PESI = Pulmonary embolism severity index; RV = right ventricular; sPESI = simplified Pulmonary embolism severity index.
aPESI Class III to V indicates moderate to very high 30-day mortality risk; sPESI ≥1 point(s) indicate high 30-day mortality risk.
bEchocardiographic criteria of RV dysfunction include RV dilation and/or an increased end-diastolic RV–LV diameter ratio (in most studies, the reported threshold value was 0.9 or 1.0); hypokinesia of the free RV wall; increased velocity of the tricuspid regurgitation jet; or combinations of the above. On computed tomographic (CT) angiography (four-chamber views of the heart), RV dysfunction is defined as an increased end-diastolic RV/LV (left ventricular) diameter ratio (with a threshold of 0.9 or 1.0).
cMarkers of myocardial injury (e.g. elevated cardiac troponin I or -T concentrations in plasma), or of heart failure as a result of (right) ventricular dysfunction (elevated natriuretic peptide concentrations in plasma).
dNeither calculation of the PESI (or sPESI) nor laboratory testing are considered necessary in patients with hypotension or shock.
ePatients in the PESI Class I–II, or with sPESI of 0, and elevated cardiac biomarkers or signs of RV dysfunction on imaging tests, are also to be classified into the intermediate-low-risk category. This might apply to situations in which imaging or biomarker results become available before calculation of the clinical severity index.
Classification of patients with acute PE based on early mortality risk
 |
 |
PE = pulmonary embolism; PESI = Pulmonary embolism severity index; RV = right ventricular; sPESI = simplified Pulmonary embolism severity index.
aPESI Class III to V indicates moderate to very high 30-day mortality risk; sPESI ≥1 point(s) indicate high 30-day mortality risk.
bEchocardiographic criteria of RV dysfunction include RV dilation and/or an increased end-diastolic RV–LV diameter ratio (in most studies, the reported threshold value was 0.9 or 1.0); hypokinesia of the free RV wall; increased velocity of the tricuspid regurgitation jet; or combinations of the above. On computed tomographic (CT) angiography (four-chamber views of the heart), RV dysfunction is defined as an increased end-diastolic RV/LV (left ventricular) diameter ratio (with a threshold of 0.9 or 1.0).
cMarkers of myocardial injury (e.g. elevated cardiac troponin I or -T concentrations in plasma), or of heart failure as a result of (right) ventricular dysfunction (elevated natriuretic peptide concentrations in plasma).
dNeither calculation of the PESI (or sPESI) nor laboratory testing are considered necessary in patients with hypotension or shock.
ePatients in the PESI Class I–II, or with sPESI of 0, and elevated cardiac biomarkers or signs of RV dysfunction on imaging tests, are also to be classified into the intermediate-low-risk category. This might apply to situations in which imaging or biomarker results become available before calculation of the clinical severity index.
Risk-adjusted management strategies in acute PE (see Table 9 for definition of the risk categories).
At the stage of clinical suspicion of PE, haemodynamically unstable patients with shock or hypotension should immediately be identified as high-risk patients (Figure 2). They require an emergency diagnostic algorithm as outlined in the previous section and, if PE is confirmed, primary pharmacological (or, alternatively, surgical or interventional) reperfusion therapy.
Patients without shock or hypotension are not at high risk of an adverse early outcome. Further risk stratification should be considered after the diagnosis of PE has been confirmed, as this may influence the therapeutic strategy and the duration of the hospitalization (see Section 5.8). In these patients, risk assessment should begin with a validated clinical prognostic score, preferably the PESI or sPESI, its simplified version, to distinguish between intermediate and low risk. Around one-third of PE patients are at low risk of an early adverse outcome as indicated by a PESI Class I or II, or a simplified PESI of 0. On the other hand, in registries and cohort studies, patients in PESI Class III–V had a 30-day mortality rate of up to 24.5%,214 and those with a simplified PESI ≥1 up to 11%.218 Accordingly, normotensive patients in PESI Class ≥III or a simplified PESI of ≥1 are considered to constitute an intermediate-risk group. Within this category, further risk assessment should be considered, focusing on the status of the RV in response to the PE-induced acute pressure overload. Patients who display evidence of both RV dysfunction (by echocardiography or CT angiography) and elevated cardiac biomarker levels in the circulation (particularly a positive cardiac troponin test) should be classified into an intermediate-high-risk category. As discussed in more detail in the following section, close monitoring is recommended in these cases to permit early detection of haemodynamic decompensation and the need for initiation of rescue reperfusion therapy.253 On the other hand, patients in whom the RV is normal on echocardiography or CT angiography and/or cardiac biomarker levels are also normal, belong to an intermediate-low-risk group.
Data from registries and cohort studies suggest that patients in PESI Class I–II, or with sPESI of 0, but with elevated cardiac biomarkers or signs of RV dysfunction on imaging tests, should also be classified into the intermediate-low-risk category.76,222,262 Nevertheless, routine performance of imaging or laboratory tests in the presence of a low PESI or a simplified PESI of 0 is not considered necessary at present as, in these cases, it has not been shown to have therapeutic implications.
Recommendations for prognostic assessment
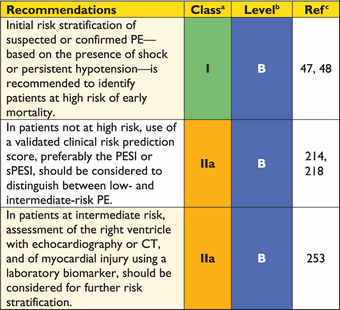 |
 |
CT = computed tomographic (pulmonary angiography); PE = pulmonary embolism; PESI = pulmonary embolism severity index; sPESI = simplified pulmonary embolism severity index.
aClass of recommendation.
bLevel of evidence.
cReferences.
5. Treatment in the acute phase
5.1 Haemodynamic and respiratory support
Acute RV failure with resulting low systemic output is the leading cause of death in patients with high-risk PE. Therefore, supportive treatment is vital in patients with PE and RV failure. Experimental studies indicate that aggressive volume expansion is of no benefit and may even worsen RV function by causing mechanical overstretch, or by reflex mechanisms that depress contractility.263 On the other hand, modest (500 mL) fluid challenge may help to increase cardiac index in patients with PE, low cardiac index, and normal BP.264
Use of vasopressors is often necessary, in parallel with (or while waiting for) pharmacological, surgical, or interventional reperfusion treatment. Norepinephrine appears to improve RV function via a direct positive inotropic effect, while also improving RV coronary perfusion by peripheral vascular alpha-receptor stimulation and the increase in systemic BP. Its use should probably be limited to hypotensive patients. Based on the results of small series, the use of dobutamine and/or dopamine may be considered for patients with PE, low cardiac index, and normal BP; however, raising the cardiac index above physiological values may aggravate the ventilation–perfusion mismatch by further redistributing flow from (partly) obstructed to unobstructed vessels.265 Epinephrine combines the beneficial properties of norepinephrine and dobutamine, without the systemic vasodilatory effects of the latter. It may therefore exert beneficial effects in patients with PE and shock.
Vasodilators decrease pulmonary arterial pressure and pulmonary vascular resistance, but the main concern is the lack of specificity of these drugs for the pulmonary vasculature after systemic (intravenous) administration. According to data from small clinical studies, inhalation of nitric oxide may improve the haemodynamic status and gas exchange of patients with PE.266,267 Preliminary data suggest that levosimendan may restore right ventricular–pulmonary arterial coupling in acute PE by combining pulmonary vasodilation with an increase in RV contractility.268
Hypoxaemia and hypocapnia are frequently encountered in patients with PE, but they are of moderate severity in most cases. A patent foramen ovale may aggravate hypoxaemia due to shunting when right atrial- exceeds left atrial pressure.80 Hypoxaemia is usually reversed with administration of oxygen. When mechanical ventilation is required, care should be taken to limit its adverse haemodynamic effects. In particular, the positive intrathoracic pressure induced by mechanical ventilation may reduce venous return and worsen RV failure in patients with massive PE; therefore, positive end-expiratory pressure should be applied with caution. Low tidal volumes (approximately 6 mL/kg lean body weight) should be used in an attempt to keep the end-inspiratory plateau pressure <30 cm H2O.
Experimental evidence suggests that extracorporeal cardiopulmonary support can be an effective procedure in massive PE.269 This notion is supported by occasional case reports and patient series.270–272
5.2 Anticoagulation
In patients with acute PE, anticoagulation is recommended, with the objective of preventing both early death and recurrent symptomatic or fatal VTE. The standard duration of anticoagulation should cover at least 3 months (also see Section 6). Within this period, acute-phase treatment consists of administering parenteral anticoagulation [unfractionated heparin (UFH), low molecular weight heparin (LMWH), or fondaparinux] over the first 5–10 days. Parenteral heparin should overlap with the initiation of a vitamin K antagonist (VKA); alternatively, it can be followed by administration of one of the new oral anticoagulants: dabigatran or edoxaban. If rivaroxaban or apixaban is given instead, oral treatment with one of these agents should be started directly or after a 1–2 day administration of UFH, LMWH or fondaparinux. In this latter case, acute-phase treatment consists of an increased dose of the oral anticoagulant over the first 3 weeks (for rivaroxaban), or over the first 7 days (for apixaban).
In some cases, extended anticoagulation beyond the first 3 months, or even indefinitely, may be necessary for secondary prevention, after weighing the individual patient's risk of recurrence vs. bleeding risk.
5.2.1 Parenteral anticoagulation
In patients with high or intermediate clinical probability for PE (see Section 3), parenteral anticoagulation should be initiated whilst awaiting the results of diagnostic tests. Immediate anticoagulation can be achieved with parenteral anticoagulants such as intravenous UFH, subcutaneous LMWH, or subcutaneous fondaparinux. LMWH or fondaparinux are preferred over UFH for initial anticoagulation in PE, as they carry a lower risk of inducing major bleeding and heparin-induced thrombocytopenia (HIT).273–276 On the other hand, UFH is recommended for patients in whom primary reperfusion is considered, as well as for those with serious renal impairment (creatinine clearance <30 mL/min), or severe obesity. These recommendations are based on the short half-life of UFH, the ease of monitoring its anticoagulant effects, and its rapid reversal by protamine. The dosing of UFH is adjusted, based on the activated partial thromboplastin time (aPTT; Web Addenda Table II).277
The LMWHs approved for the treatment of acute PE are listed in Table 10. LMWH needs no routine monitoring, but periodic measurement of anti-factor Xa activity (anti-Xa levels) may be considered during pregnancy.279 Peak values of anti-factor Xa activity should be measured 4 hours after the last injection and trough values just before the next dose of LMWH would be due; the target range is 0.6–1.0 IU/mL for twice-daily administration, and 1.0–2.0 IU/mL for once-daily administration.280
Low molecular weight heparin and pentasaccharide (fondaparinux) approved for the treatment of pulmonary embolism
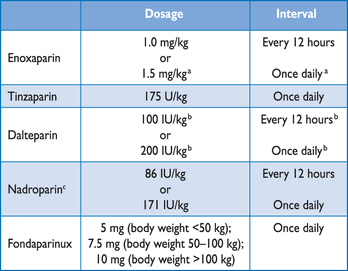 |
 |
All regimens administered subcutaneously.
IU = international units; LMWH = low molecular weight heparin.
aOnce-daily injection of enoxaparin at the dosage of 1.5 mg/kg is approved for inpatient (hospital) treatment of PE in the United States and in some, but not all, European countries.
bIn cancer patients, dalteparin is given at a dose of 200 IU/kg body weight (maximum, 18 000 IU) once daily over a period of 1 month, followed by 150 IU/kg once daily for 5 months.278 After this period, anticoagulation with a vitamin K antagonist or a LMWH should be continued indefinitely or until the cancer is considered cured.
cNadroparin is approved for treatment of PE in some, but not all, European countries.
Low molecular weight heparin and pentasaccharide (fondaparinux) approved for the treatment of pulmonary embolism
 |
 |
All regimens administered subcutaneously.
IU = international units; LMWH = low molecular weight heparin.
aOnce-daily injection of enoxaparin at the dosage of 1.5 mg/kg is approved for inpatient (hospital) treatment of PE in the United States and in some, but not all, European countries.
bIn cancer patients, dalteparin is given at a dose of 200 IU/kg body weight (maximum, 18 000 IU) once daily over a period of 1 month, followed by 150 IU/kg once daily for 5 months.278 After this period, anticoagulation with a vitamin K antagonist or a LMWH should be continued indefinitely or until the cancer is considered cured.
cNadroparin is approved for treatment of PE in some, but not all, European countries.
Fondaparinux is a selective factor Xa inhibitor administered once daily by subcutaneous injection at weight-adjusted doses, without the need for monitoring (Table 10). In patients with acute PE and no indication for thrombolytic therapy, fondaparinux was associated with recurrent VTE and major bleeding rates similar to those obtained with intravenous UFH.281 No proven cases of HIT have been reported with fondaparinux.282 Subcutaneous fondaparinux is contraindicated in patients with severe renal insufficiency (creatinine clearance <30 mL/min) because it will accumulate and increase the risk of haemorrhage. Accumulation also occurs in patients with moderate renal insufficiency (clearance 30–50 mL/min) and, therefore, the dose should be reduced by 50% in these patients.283
5.2.2 Vitamin K antagonists
Oral anticoagulants should be initiated as soon as possible, and preferably on the same day as the parenteral anticoagulant. VKAs have been the ‘gold standard' in oral anticoagulation for more than 50 years and warfarin, acenocoumarol, phenprocoumon, phenindione and flunidione remain the predominant anticoagulants prescribed for PE.284 Anticoagulation with UFH, LMWH, or fondaparinux should be continued for at least 5 days and until the international normalized ratio (INR) has been 2.0–3.0 for two consecutive days.285
Warfarin can be started at a dose of 10 mg in younger (e.g. <60 years of age), otherwise healthy outpatients, and at a dose of 5 mg in older patients and in those who are hospitalized. The daily dose is adjusted according to the INR over the next 5–7 days, aiming for an INR level of 2.0–3.0. Rapid-turnaround pharmacogenetic testing may increase the precision of warfarin dosing.286,287 In particular, variations in two genes may account for more than one-third of the dosing variability of warfarin. One gene determines the activity of cytochrome CYP2C9, the hepatic isoenzyme that metabolizes the S-enantiomer of warfarin into its inactive form, while the other determines the activity of vitamin K epoxide reductase, the enzyme that produces the active form of vitamin K.288 Pharmacogenetic algorithms incorporate genotype and clinical information and recommend warfarin doses according to integration of these data. A trial published in 2012 indicated that, compared with standard care, pharmacogenetic guidance of warfarin dosing resulted in a 10% absolute reduction in out-of-range INRs at one month, primarily due to fewer INR values <1.5; this improvement coincided with a 66% lower rate of DVT.289 In 2013, three large randomized trials were published.290–292 All used, as the primary endpoint, the percentage of time in therapeutic range (TTR) (a surrogate for the quality of anticoagulation) for the INR during the first 4–12 weeks of therapy. In 455 patients, genotype-guided doses of warfarin, with a point-of-care test, resulted in a significant but modest increase in TTR over the first 12 weeks, compared with a fixed 3-day loading-dose regimen (67.4% vs. 60.3%; P < 0.001). The median time to reaching a therapeutic INR was reduced from 29 to 21 days.292 Another study in 1015 patients compared warfarin loading—based on genotype data in combination with clinical variables—with a loading regimen based on the clinical data alone; no significant improvement was found in either group in terms of the TTR achieved between days 4 and 28 of therapy.291 Lack of improvement was also shown by a trial involving 548 patients, comparing acenocoumarol or phenprocoumon loading—based on point-of-care genotyping in combination with clinical variables (age, sex, height, weight, amiodarone use)—with a loading regimen based entirely on clinical information.290
In summary, the results of recent trials appear to indicate that pharmacogenetic testing, used on top of clinical parameters, does not improve the quality of anticoagulation. They also suggest that dosing based on the patient's clinical data is possibly superior to fixed loading regimens, and they point out the need to place emphasis on improving the infrastructure of anticoagulation management by optimizing the procedures that link INR measurement with provision of feedback to the patient and individually tailoring dose adjustments.
5.2.3 New oral anticoagulants
The design and principal findings of phase III clinical trials on the acute-phase treatment and standard duration of anticoagulation after PE or VTE with non-vitamin K-dependent new oral anticoagulants (NOACs) are summarized in Table 11. In the RE-COVER trial, the direct thrombin inhibitor dabigatran was compared with warfarin for the treatment of VTE.293 The primary outcome was the 6-month incidence of recurrent, symptomatic, objectively confirmed VTE. Overall, 2539 patients were enrolled, 21% with PE only and 9.6% with PE plus DVT. Parenteral anticoagulation was administered for a mean of 10 days in both groups. With regard to the efficacy endpoint, dabigatran was non-inferior to warfarin (HR 1.10; 95% CI 0.65–1.84). No significant differences were observed with regard to major bleeding episodes (Table 11), but there were fewer episodes of any bleeding with dabigatran (HR 0.71; 95% CI 0.59–0.85). Its twin study, RE-COVER II,294 enrolled 2589 patients and confirmed these results (primary efficacy outcome: HR 1.08; 95% CI 0.64-1.80; major bleeding: HR 0.69; 95% CI 0.36-1.32) (Table 11). For the pooled RE-COVER population, the HR for efficacy was 1.09 (95% CI 0.76-1.57) and for major bleeding 0.73 (95% CI 0.48-1.11).294
Overview of phase III clinical trials with non-vitamin K-dependent new oral anticoagulants (NOACs) for the acute-phase treatment and standard duration of anticoagulation after VTE
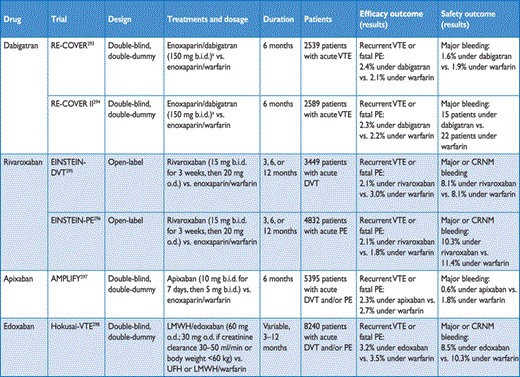 |
 |
b.i.d. = bis in die (twice daily); CRNM = clinically relevant non-major; DVT= deep vein thrombosis; o.d. = omni die (once daily); PE= pulmonary embolism; UFH = unfractionated heparin; VTE = venous thromboembolism.
a Approved doses of dabigatran are 150 mg b.i.d. and 110 mg b.i.d.
Overview of phase III clinical trials with non-vitamin K-dependent new oral anticoagulants (NOACs) for the acute-phase treatment and standard duration of anticoagulation after VTE
 |
 |
b.i.d. = bis in die (twice daily); CRNM = clinically relevant non-major; DVT= deep vein thrombosis; o.d. = omni die (once daily); PE= pulmonary embolism; UFH = unfractionated heparin; VTE = venous thromboembolism.
a Approved doses of dabigatran are 150 mg b.i.d. and 110 mg b.i.d.
In the EINSTEIN-DVT and EINSTEIN-PE trials,295,296 single oral drug treatment with the direct factor Xa inhibitor rivaroxaban (15 mg twice daily for 3 weeks, followed by 20 mg once daily) was tested against enoxaparin/warfarin in patients with VTE using a randomized, open-label, non-inferiority design. In particular, EINSTEIN-PE enrolled 4832 patients who had acute symptomatic PE, with or without DVT. Rivaroxaban was non-inferior to standard therapy for the primary efficacy outcome of recurrent symptomatic VTE (HR 1.12; 95% CI 0.75–1.68). The principal safety outcome [major or clinically relevant non-major (CRNM) bleeding] occurred with similar frequency in the two treatment groups (HR for rivaroxaban, 0.90; 95% CI 0.76–1.07) (Table 11), but major bleeding was less frequent in the rivaroxaban group, compared with the standard-therapy group (1.1% vs. 2.2%, HR 0.49; 95% CI 0.31–0.79).
The Apixaban for the Initial Management of Pulmonary Embolism and Deep-Vein Thrombosis as First-line Therapy (AMPLIFY) study compared single oral drug treatment using the direct factor Xa inhibitor apixaban (10 mg twice daily for 7 days, followed by 5 mg b.i.d.) with conventional therapy (enoxaparin/warfarin) in 5395 patients with acute VTE, 1836 of whom presented with PE (Table 11).297 The primary efficacy outcome was recurrent symptomatic VTE or death related to VTE. The principal safety outcomes were major bleeding alone, and major bleeding plus CRNM bleeding. Apixaban was non-inferior to conventional therapy for the primary efficacy outcome (relative risk [RR] 0.84; 95% CI 0.60–1.18). Major bleeding occurred less frequently under apixaban compared with conventional therapy (RR 0.31; 95% CI 0.17–0.55; P < 0.001 for superiority) (Table 11). The composite outcome of major bleeding and CRNM bleeding occurred in 4.3% of the patients in the apixaban group, compared with 9.7% of those in the conventional-therapy group (RR 0.44; 95% CI 0.36–0.55; P < 0.001).
The HokusaI–VTE study compared the direct factor Xa inhibitor edoxaban with conventional therapy in 8240 patients with acute VTE (3319 of whom presented with PE) who had initially received heparin for at least 5 days (Table 11).298 Patients received edoxaban at a dose of 60 mg once daily (reduced to 30 mg once daily in the case of creatinine clearance of 30–50 mL/min or a body weight <60 kg), or warfarin. The study drug was administered for 3–12 months; all patients were followed up for 12 months. Edoxaban was non-inferior to warfarin with respect to the primary efficacy outcome of recurrent symptomatic VTE or fatal PE (HR 0.89; 95% CI 0.70–1.13). The principal safety outcome, major or CRNM bleeding, occurred less frequently in the edoxaban group (HR 0.81; 95% CI 0.71–0.94; P = 0.004 for superiority) (Table 11). In 938 patients who presented with acute PE and elevated NT-proBNP concentrations (≥500 pg/mL), the rate of recurrent VTE was 3.3% in the edoxaban group and 6.2% in the warfarin group (HR 0.52; 95% CI 0.28–0.98).
In summary, the results of the trials using NOACs in the treatment of VTE indicate that these agents are non-inferior (in terms of efficacy) and possibly safer (particularly in terms of major bleeding) than the standard heparin/VKA regimen.299 High TTR values were achieved under VKA treatment in all trials; on the other hand, the study populations included relatively young patients, very few of whom had cancer. At present, NOACs can be viewed as an alternative to standard treatment. At the moment of publication of these guidelines, rivaroxaban, dabigatran and apixaban are approved for treatment of VTE in the European Union; edoxaban is currently under regulatory review. Experience with NOACs is still limited but continues to accumulate. Practical recommendations for the handling of NOACs in different clinical scenarios and the management of their bleeding complications have recently been published by the European Heart Rhythm Association.300
5.3 Thrombolytic treatment
Thrombolytic treatment of acute PE restores pulmonary perfusion more rapidly than anticoagulation with UFH alone.301,302 The early resolution of pulmonary obstruction leads to a prompt reduction in pulmonary artery pressure and resistance, with a concomitant improvement in RV function.302 The haemodynamic benefits of thrombolysis are confined to the first few days; in survivors, differences are no longer apparent at one week after treatment.301,303,304
The approved regimens of thrombolytic agents for PE are shown in Web Addenda Table III; the contraindications to thrombolysis are displayed in Web Addenda Table IV. Accelerated regimens administered over 2 hours are preferable to prolonged infusions of first-generation thrombolytic agents over 12–24 hours.305–308 Reteplase and desmoteplase have been tested against recombinant tissue plasminogen activator (rtPA) in acute PE, with similar results in terms of haemodynamic parameters;309,310 tenecteplase was tested against placebo in patients with intermediate-risk PE.253,303,311 At present, none of these agents is approved for use in PE.
Unfractionated heparin infusion should be stopped during administration of streptokinase or urokinase; it can be continued during rtPA infusion. In patients receiving LMWH or fondaparinux at the time that thrombolysis is initiated, infusion of UFH should be delayed until 12 hours after the last LMWH injection (given twice daily), or until 24 hours after the last LMWH or fondaparinux injection (given once daily). Given the bleeding risks associated with thrombolysis and the possibility that it may become necessary to immediately discontinue or reverse the anticoagulant effect of heparin, it appears reasonable to continue anticoagulation with UFH for several hours after the end of thrombolytic treatment before switching to LMWH or fondaparinux.
Overall, >90% of patients appear to respond favourably to thrombolysis, as judged by clinical and echocardiographic improvement within 36 hours.313 The greatest benefit is observed when treatment is initiated within 48 hours of symptom onset, but thrombolysis can still be useful in patients who have had symptoms for 6–14 days.314
A review of randomized trials performed before 2004 indicated that thrombolysis may be associated with a reduction in mortality or recurrent PE in high-risk patients who present with haemodynamic instability.168 In a recent epidemiological report, in-hospital mortality attributable to PE was lower in unstable patients who received thrombolytic therapy, compared with those who did not (RR 0.20; 95% CI 0.19–0.22; P<0.0001).315 Most contraindications to thrombolysis (Web Addenda Table IV) should be considered relative in patients with life-threatening, high-risk PE.
In the absence of haemodynamic compromise at presentation, the clinical benefits of thrombolysis have remained controversial for many years. In a randomized comparison of heparin vs. alteplase in 256 normotensive patients with acute PE and evidence of RV dysfunction or pulmonary hypertension—obtained by clinical examination, echocardiography, or right heart catheterization—thrombolytic treatment (mainly secondary thrombolysis) reduced the incidence of escalation to emergency treatment (from 24.6% to 10.2%; P = 0.004), without affecting mortality.252 More recently, the Pulmonary Embolism Thrombolysis (PEITHO) trial was published.253 This was a multicentre, randomized, double-blind comparison of thrombolysis with a single weight-adapted intravenous bolus of tenecteplase plus heparin vs. placebo plus heparin. Patients with acute PE were eligible for the study if they had RV dysfunction, confirmed by echocardiography or CT angiography, and myocardial injury confirmed by a positive troponin I or -T test. A total of 1006 patients were enrolled. The primary efficacy outcome, a composite of all-cause death or haemodynamic decompensation/collapse within 7 days of randomization, was significantly reduced with tenecteplase (2.6% vs. 5.6% in the placebo group; P = 0.015; OR 0.44; 95% CI 0.23–0.88). The benefit of thrombolysis was mainly driven by a significant reduction in the rate of haemodynamic collapse (1.6% vs. 5.0%; P = 0.002); all-cause 7-day mortality was low: 1.2% in the tenecteplase group and 1.8% in the placebo group (P = 0.43). In another randomized study comparing LMWH alone vs. LMWH plus an intravenous bolus of tenecteplase in intermediate-risk PE, patients treated with tenecteplase had fewer adverse outcomes, better functional capacity, and greater quality of life at 3 months.311
Thrombolytic treatment carries a risk of major bleeding, including intracranial haemorrhage. Analysis of pooled data from trials using various thrombolytic agents and regimens reported intracranial bleeding rates between 1.9% and 2.2%.316,317 Increasing age and the presence of comorbidities have been associated with a higher risk of bleeding complications.318 The PEITHO trial showed a 2% incidence of haemorrhagic stroke after thrombolytic treatment with tenecteplase (versus 0.2% in the placebo arm) in patients with intermediate-high-risk PE. Major non-intracranial bleeding events were also increased in the tenecteplase group, compared with placebo (6.3% vs. 1.5%; P < 0.001).253 These results underline the need to improve the safety of thrombolytic treatment in patients at increased risk of intracranial or other life-threatening bleeding. A strategy using reduced-dose rtPA appeared to be safe in the setting of ‘moderate’ PE in a study that included 121 patients,319 and another trial on 118 patients with haemodynamic instability or ‘massive pulmonary obstruction’ reported similar results.320 An alternative approach may consist of local, catheter-delivered, ultrasound-assisted thrombolysis using small doses of a thrombolytic agent. (See Section 5.5.)
In patients with mobile right heart thrombi, the therapeutic benefits of thrombolysis remain controversial. Good results were reported in some series,199,200 but in other reports short-term mortality exceeded 20% despite thrombolysis.184,321,322
5.4 Surgical embolectomy
The first successful surgical pulmonary embolectomy was performed in 1924, several decades before the introduction of medical treatment for PE. Multidisciplinary teams enjoying the early and active involvement of cardiac surgeons have recently reintroduced the concept of surgical embolectomy for high-risk PE, and also for selected patients with intermediate-high-risk PE, particularly if thrombolysis is contraindicated or has failed. Surgical embolectomy has also been successfully performed in patients with right heart thrombi straddling the interatrial septum through a patent foramen ovale.323,324
Pulmonary embolectomy is technically a relatively simple operation. The site of surgical care does not appear to have a significant effect on operative outcomes, and thus patients need not be transferred to a specialized cardiothoracic centre if on-site embolectomy using extracorporeal circulation is possible.325 Transportable extracorporeal assistance systems with percutaneous femoral cannulation can be helpful in critical situations, ensuring circulation and oxygenation until definitive diagnosis.326,327 Following rapid transfer to the operating room and induction of anaesthesia and median sternotomy, normothermic cardiopulmonary bypass should be instituted. Aortic cross-clamping and cardioplegic cardiac arrest should be avoided.328 With bilateral PA incisions, clots can be removed from both pulmonary arteries down to the segmental level under direct vision. Prolonged periods of post-operative cardiopulmonary bypass and weaning may be necessary for recovery of RV function.
With a rapid multidisciplinary approach and individualized indications for embolectomy before haemodynamic collapse, perioperative mortality rates of 6% or less have been reported.326,328–330 Pre-operative thrombolysis increases the risk of bleeding, but it is not an absolute contraindication to surgical embolectomy.331
Over the long term, the post-operative survival rate, World Health Organization functional class, and quality of life were favourable in published series.327,329,332,333
Patients presenting with an episode of acute PE superimposed on a history of long-lasting dyspnoea and pulmonary hypertension are likely to suffer from chronic thromboembolic pulmonary hypertension. These patients should be transferred to an expert centre for pulmonary endarterectomy (see Section 7).
5.5 Percutaneous catheter-directed treatment
The objective of interventional treatment is the removal of obstructing thrombi from the main pulmonary arteries to facilitate RV recovery and improve symptoms and survival.169 For patients with absolute contraindications to thrombolysis, interventional options include (i) thrombus fragmentation with pigtail or balloon catheter, (ii) rheolytic thrombectomy with hydrodynamic catheter devices, (iii) suction thrombectomy with aspiration catheters and (iv) rotational thrombectomy. On the other hand, for patients without absolute contraindications to thrombolysis, catheter-directed thrombolysis or pharmacomechanical thrombolysis are preferred approaches. An overview of the available devices and techniques for percutaneous catheter-directed treatment of PE is given in Web Addenda Table V.169,334
A review on interventional treatment included 35 non-randomized studies covering 594 patients.334 Clinical success, defined as stabilization of haemodynamic parameters, resolution of hypoxia, and survival to discharge, was 87%. The contribution of the mechanical catheter intervention per se to clinical success is unclear because 67% of patients also received adjunctive local thrombolysis. Publication bias probably resulted in underreporting of major complications (reportedly affecting 2% of interventions), which may include death from worsening RV failure, distal embolization, pulmonary artery perforation with lung haemorrhage, systemic bleeding complications, pericardial tamponade, heart block or bradycardia, haemolysis, contrast-induced nephropathy, and puncture-related complications.169
While anticoagulation with heparin alone has little effect on improvement of RV size and performance within the first 24–48 hours,304 the extent of early RV recovery after low-dose catheter-directed thrombolysis appears comparable to that after standard-dose systemic thrombolysis.303,335 In a randomized, controlled clinical trial of 59 intermediate-risk patients, when compared with treatment by heparin alone, catheter-directed ultrasound-accelerated thrombolysis—administering 10 mg t-PA per treated lung over 15 hours—significantly reduced the subannular RV/LV dimension ratio between baseline and 24-hour follow-up without an increase in bleeding complications.336
5.6 Venous filters
Venous filters are usually placed in the infrarenal portion of the inferior vena cava (IVC). If a thrombus is identified in the renal veins, suprarenal placement may be indicated. Venous filters are indicated in patients with acute PE who have absolute contraindications to anticoagulant drugs, and in patients with objectively confirmed recurrent PE despite adequate anticoagulation treatment. Observational studies suggest that insertion of a venous filter might reduce PE-related mortality rates in the acute phase,337,338 benefit possibly coming at the cost of an increased risk of recurrence of VTE.338
Complications associated with permanent IVC filters are common, although they are rarely fatal.339 Overall, early complications—which include insertion site thrombosis—occur in approximately 10% of patients. Placement of a filter in the superior vena cava carries the risk of pericardial tamponade.340 Late complications are more frequent and include recurrent DVT in approximately 20% of patients and post-thrombotic syndrome in up to 40%. Occlusion of the IVC affects approximately 22% of patients at 5 years and 33% at 9 years, regardless of the use and duration of anticoagulation.341,342
Eight-year follow-up of a randomized study on 400 patients with DVT (with or without PE), all of whom had initially received anticoagulant treatment for at least 3 months, showed that patients undergoing permanent IVC filter insertion had a reduced risk of recurrent PE—at the cost of an increased risk of recurrent DVT—and no overall effect on survival.341
Non-permanent IVC filters are classified as temporary or retrievable devices. Temporary filters must be removed within few days, while retrievable filters can be left in place for longer periods. When non-permanent filters are used, it is recommended that they be removed as soon as it is safe to use anticoagulants. Despite this, they are often left in situ for longer periods, with a late complication rate of at least 10%; this includes filter migration, tilting or deformation, penetration of the cava wall by filter limbs, fracturing of the filter and embolization of fragments, and thrombosis of the device.343,344
There are no data to support the routine use of venous filters in patients with free-floating thrombi in the proximal veins; in one series, among PE patients who received adequate anticoagulant treatment alone (without a venous filter), the recurrence rate was low (3.2%).345 There is also no evidence to support the use of IVC filters in patients scheduled for systemic thrombolysis, surgical embolectomy, or pulmonary thrombendarterectomy.
5.7 Early discharge and home treatment
When considering early discharge and outpatient treatment of patients with acute PE, the crucial issue is to select those patients who are at low risk of an adverse early outcome. A number of risk-prediction models have been developed (see Section 4).346 Of these, the PESI (Table 7) is the most extensively validated score to date.211–214 One randomized trial employed a low (Class I or II) PESI as one of the inclusion criteria for home treatment of acute PE.217 The simplified form of this index (sPESI) possesses a high sensitivity for identification of low-risk PE,76,221 but its value for selecting candidates for early discharge and home treatment has not yet been directly investigated.
The Hestia criteria comprise a set of clinical parameters that can easily be obtained at the bedside. In a single-arm management trial that used these criteria to select candidates for home treatment, the rate of recurrent VTE was 2.0% (0.8–4.3%) in patients with acute PE who were discharged within 24 hours.347 The Hestia criteria have not yet been externally validated.
The value of NT-proBNP as a laboratory biomarker for selecting candidates for home treatment has been evaluated in a single-arm management study in which, out of 152 patients (upper margin of the 95% CI: 2.4%) with clinically defined very low-risk PE and BNP levels <500 pg/mL, none died or suffered recurrence of VTE or major bleeding complications during three-month follow-up.237 The value of imaging procedures (echocardiography or CT scan) for excluding RV dysfunction before early discharge has not been investigated in clinical outcome trials.
Table 12 summarizes the designs of the recent multicentre clinical trials that investigated the three-month clinical outcome of patients with PE, who were discharged early or treated entirely as outpatients. Overall, the proportion of screened patients who were identified as eligible for home treatment ranged from 13–51%.348 Two of the studies were randomized, one allocating patients to receive either in-hospital treatment for only 3 days (followed by discharge) or a ‘full’ hospital stay,349 while the other assigned them to receive anticoagulation either entirely outside the hospital (patients were discharged within 24 hours) or partly in hospital.217 The first of these studies, in which a prospectively developed prediction rule was used to define low risk, was terminated prematurely because of an increased short-term mortality rate in the early-discharge arm; two patients (2.8%) in this arm died early, one from gastrointestinal bleeding and the other as a result of cardiac arrest in the presence of a right heart thrombus. Overall mortality was 4.2% in the early-discharge arm, as against 8.3% in the hospitalization arm.349 In the second trial, which was larger, there was one non-VTE related death in each treatment group (0.6%); one patient in the outpatient arm (0.6%) but none in the hospital group suffered non-fatal recurrent VTE.217 In a meta-analysis of 14 (mostly cohort-) studies, the pooled incidences of recurrent VTE, major bleeding and total mortality did not differ significantly between outpatients, patients discharged early, and those treated as inpatients.351
Design of recent multicentre trials on home treatment of acute PE (modified from (348))
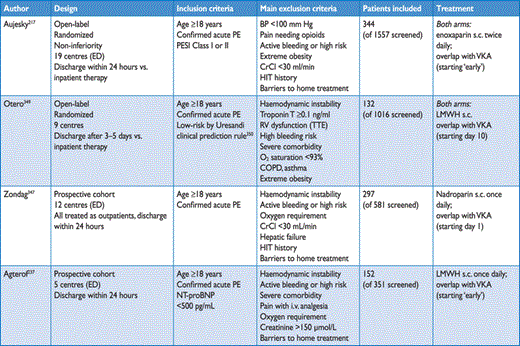 |
 |
BP = (systolic) blood pressure; COPD = (severe) chronic obstructive pulmonary disease; CrCl = creatinine clearance; ED = emergency department(s); HIT = heparin-induced thrombocytopenia; i.v. = intravenous; LMWH = low molecular weight heparin; NT-proBNP = N-terminal pro-brain natriuretic peptide; PE = pulmonary embolism; PESI = Pulmonary embolism severity index (see Table 7); RV = right ventricular; s.c. = subcutaneous; TTE = transthoracic echocardiography; VKA = vitamin K antagonist.
Design of recent multicentre trials on home treatment of acute PE (modified from (348))
 |
 |
BP = (systolic) blood pressure; COPD = (severe) chronic obstructive pulmonary disease; CrCl = creatinine clearance; ED = emergency department(s); HIT = heparin-induced thrombocytopenia; i.v. = intravenous; LMWH = low molecular weight heparin; NT-proBNP = N-terminal pro-brain natriuretic peptide; PE = pulmonary embolism; PESI = Pulmonary embolism severity index (see Table 7); RV = right ventricular; s.c. = subcutaneous; TTE = transthoracic echocardiography; VKA = vitamin K antagonist.
5.8 Therapeutic strategies
An algorithm of the recommended therapeutic strategies for acute PE is shown in Figure 5.
5.8.1 Pulmonary embolism with shock or hypotension (high-risk pulmonary embolism)
Patients with PE presenting with shock or hypotension are at high risk of in-hospital death, particularly during the first few hours after admission. Besides haemodynamic and respiratory support, intravenous UFH should be administered to these patients as the preferred mode of initial anticoagulation, as LMWH or fondaparinux have not been tested in the setting of hypotension and shock.
Primary reperfusion treatment, particularly systemic thrombolysis, is the treatment of choice for patients with high-risk PE. In patients with contraindications to thrombolysis—and in those in whom thrombolysis has failed to improve the haemodynamic status—surgical embolectomy is recommended if surgical expertise and resources are available. As an alternative to surgery, percutaneous catheter-directed treatment should be considered if expertise with this method and the appropriate resources are available on site. In these cases, treatment decisions should be made by an interdisciplinary team involving a thoracic surgeon or interventional cardiologist, as appropriate.
5.8.2 Pulmonary embolism without shock or hypotension (intermediate- or low-risk pulmonary embolism)
For most cases of acute PE without haemodynamic compromise, LMWH or fondaparinux, given subcutaneously at weight-adjusted doses without monitoring, is the treatment of choice unless there is severe renal dysfunction.
Patients not suffering from shock or hypotension require further risk stratification after the diagnosis of PE has been confirmed. In these patients, risk assessment should begin with a validated clinical score, preferably the PESI or sPESI.
Low-risk patients in the PESI Class I or II, and probably those with sPESI of 0 (Table 9), should be considered for early discharge and outpatient treatment, if this appears feasible based on the patient's anticipated compliance as well as his/her family and social background. For all other patients, assessment of RV function by echocardiography (or CT angiography) and cardiac troponin testing should be considered.
Based on the results of a recently published randomized trial,253 and as explained in the section on prognostic assessment, patients with acute PE, an echocardiogram or CT scan indicating RV dysfunction, and a positive cardiac troponin test belong to an intermediate-high-risk group (Table 9). Full-dose systemic thrombolytic therapy, given as primary reperfusion therapy, can prevent potentially life-threatening haemodynamic decompensation or collapse in these patients, but this benefit is counterbalanced by a high risk of haemorrhagic stroke or major non-intracranial bleeding.253 Accordingly, systemic thrombolysis is not routinely recommended as primary treatment for patients with intermediate-high-risk PE, but should be considered if clinical signs of haemodynamic decompensation appear. Surgical pulmonary embolectomy or percutaneous catheter-directed treatment may be considered as alternative, ‘rescue’ procedures for patients with intermediate-high-risk PE, in whom haemodynamic decompensation appears imminent and the anticipated bleeding risk under systemic thrombolysis is high.
Other laboratory markers, such as BNP, NT-proBNP and H-FABP, have also been shown to possess additive prognostic value to clinical and imaging parameters in cohort studies; their potential therapeutic implications have not yet been investigated in prospective trials.
Normotensive patients in the PESI Class III or higher, or sPESI of at least 1, in whom the echocardiogram (or CT angiogram) or the cardiac troponin test—or both—are normal, belong to an intermediate-low-risk group. Anticoagulation is indicated. Existing evidence does not support primary reperfusion treatment. There is no evidence to suggest that bed rest has any beneficial effect on these patients' clinical outcome.
5.9 Areas of uncertainty
Although a large number of recent cohort studies have helped to further refine risk stratification in not-high-risk patients with confirmed PE, the clinical implications of prognostic assessment—and in particular the therapeutic strategy for patients at intermediate-high risk—warrant further investigation. It will be necessary to elaborate on (i) whether reduced-dose intravenous thrombolysis is indeed safe and effective and (ii) whether catheter-directed treatment can evolve to become a widely available (and affordable) alternative option. The results of the completed large phase III trials on the use of new oral anticoagulants in the treatment of PE and secondary prevention of VTE appear convincing and confirm that the breakthrough in anticoagulation therapy has extended to include VTE. Nevertheless, the accumulation of clinical experience with these drugs under ‘real world’ conditions will have to proceed at a prudent pace. Finally, further management trials are necessary to ‘crystallize' the criteria that might permit early discharge and home treatment of low-risk patients with acute PE.
Recommendations for acute phase treatment
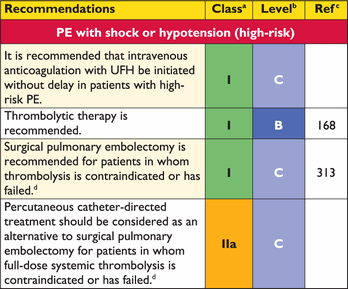 |
 |
PE = pulmonary embolism; UFH = unfractionated heparin.
aClass of recommendation.
bLevel of evidence.
cReferences.
dIf appropriate expertise and resources are available on site.
Recommendations for acute phase treatment
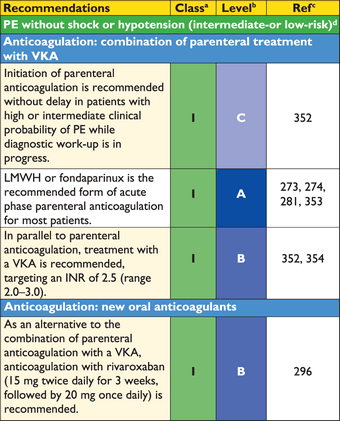 |
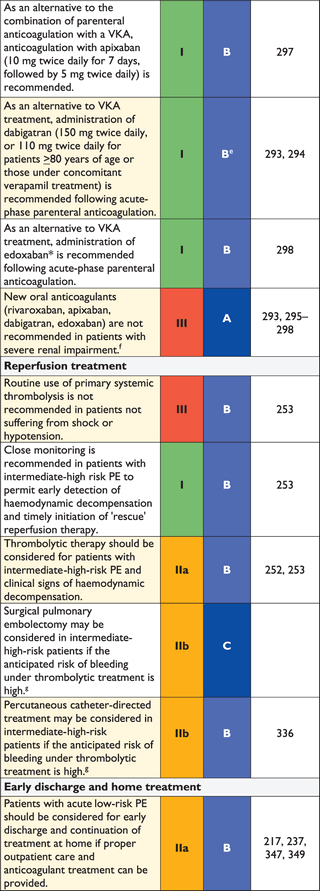 |
 |
 |
* CAUTION: Edoxaban is currently subject to regulatory review for the treatment of venous thromboembolism in the European Union.
aPTT = activated partial thromboplastin time; INR = international normalized ratio; LMWH = low molecular weight heparin; PE = pulmonary embolism; UFH = unfractionated heparin; VKA = vitamin K antagonist.
aClass of recommendation.
bLevel of evidence.
cReferences.
dSee Table 9 for definition of the risk categories.
eRE-COVER and RE-COVER II are considered one large trial.
fCreatinine clearance <30 mL/min for rivaroxaban, dabigatran and edoxaban; and <25 mL/min for apixaban.
gIf appropriate expertise and resources are available on site.
Recommendations for venous filters
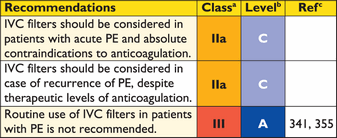 |
 |
IVC = inferior vena cava; PE = pulmonary embolism.
aClass of recommendation.
bLevel of evidence.
cReferences.
6. Duration of anticoagulation
The aim of anticoagulant treatment in patients with PE is to prevent recurrence of VTE. VKAs are used in most cases, while LMWH is preferred in patients with VTE and cancer.356,357 Three new oral anticoagulant agents have been evaluated in the extended treatment of VTE.
Most of the studies focusing on long-term anticoagulation for VTE have included patients with DVT, with or without PE, and only one study specifically focussed on patients with PE.358 The incidence of recurrent VTE does not depend on the clinical manifestation of the first event (i.e. it is similar after PE and after DVT); however, in patients who have suffered PE, VTE more frequently recurs as symptomatic PE while, in patients who have suffered DVT, it tends to recur more frequently as DVT.359
Clinical trials have evaluated various durations of anticoagulant treatment for VTE. The main findings of these studies were (i) patients with PE should receive at least 3 months of anticoagulant treatment, (ii) after withdrawal of anticoagulant treatment, the risk of recurrence if anticoagulants are stopped after 6 or 12 months can be expected to be similar to that after 3 months and (iii) indefinite treatment reduces the risk for recurrent VTE by about 90%, but this benefit is partially offset by a 1% or higher annual risk of major bleeding.360–363 In general, VKAs are highly effective in preventing recurrent VTE during treatment, but they do not eliminate the risk of subsequent recurrence after discontinuation of treatment.361,362 Thus, anticoagulants are discontinued when the perceived risk of anticoagulation-related bleeding and the inconvenience of remaining on treatment outweigh the risk of recurrent VTE.
Active cancer is a major risk factor for recurrence of VTE, the rate of recurrence being approximately 20% during the first 12 months after the index event.364,365 Therefore, patients with cancer are candidates for indefinite anticoagulant treatment after an initial episode of PE. In a randomized trial of patients with DVT and cancer, the LMWH dalteparin, given at the dose of 200 U/kg once daily for 4–6 weeks, followed by 75% of the initial dose given once daily for up to 6 months, was more effective than warfarin in preventing recurrent VTE.278 Accordingly, at least 3–6 months of treatment with LMWH are recommended for patients with VTE and cancer (see Section 8.2). The optimal treatment to be given after the first 6 months is less clear but treatment with LMWH or VKA is recommended as long as the disease is considered active.
With the exception of patients with cancer, the risk for recurrent VTE after discontinuation of treatment is related to the features of the index VTE event. A study that followed patients with a first episode of acute PE found that the recurrence rate after discontinuation of treatment was approximately 2.5% per year after PE associated with reversible risk factors compared with 4.5% per year after unprovoked PE.358 Similar observations were made in other prospective studies in patients with DVT.360 Recurrence rates may be higher, up to 10%, in the first year after withdrawal of anticoagulant treatment. As mentioned in the Introduction, VTE is held to be ‘provoked’ in the presence of a temporary or reversible risk factor (such as surgery, trauma, immobilization, pregnancy, oral contraceptive use or hormone replacement therapy) at the time of diagnosis, and ‘unprovoked’ in the absence thereof. For patients with provoked PE, treatment with a VKA for 3 months is preferable to a shorter period. Treatment for longer than 3 months is generally not recommended, provided that the transient risk factor no longer exists.358
The assessment of the risk of recurrence in patients with unprovoked PE is more complex.54–56 The following risk factors may help to identify patients at higher long-term relative risk of recurrence (1.5–2.0): (i) one or more previous episodes of VTE, (ii) antiphospholipid antibody syndrome, (iii) hereditary thrombophilia and (iv) residual thrombosis in the proximal veins. An additional risk factor for recurrence after PE was reported to be the persistence of RV dysfunction at hospital discharge as assessed by echocardiography.366 On the other hand, a negative D-dimer test one month after withdrawal of VKA seems to be a protective factor for recurrence of VTE (RR 0.4).367
Among carriers of molecular thrombophilia, patients with lupus anticoagulant, those with a confirmed deficit of protein C or protein S, and patients with homozygous factor V Leiden or homozygous prothrombin G20210A (PTG20210A), may be candidates for indefinite anticoagulant treatment after a first unprovoked VTE. No evidence of the clinical benefit of extended anticoagulant treatment is currently available for carriers of heterozygous factor V Leiden or PTG20210A.
There are no properly evaluated bleeding risk scores for patients receiving anticoagulant treatment for VTE. Based on currently available evidence, risk factors include (i) old age (particularly >75 years), (ii) previous gastrointestinal bleeding (particularly if not associated with a reversible or treatable cause), (iii) previous stroke, either haemorrhagic or ischaemic, (iv) chronic renal or hepatic disease, (v) concomitant antiplatelet therapy (to be avoided, if possible), (vi) other serious acute or chronic illness, (vii) poor anticoagulation control and (viii) sub-optimal monitoring of anticoagulant therapy.
Based on the balance between the risk of recurrence of VTE and that of bleeding, patients with unprovoked PE should be treated with VKA for at least 3 months. After this period, indefinite anticoagulation therapy should be considered for patients with a first unprovoked proximal DVT or PE and a low risk of bleeding, provided that this is consistent with the patient's preference. Notably, the term ‘indefinite anticoagulation’ is not synonymous with ‘lifelong treatment’; it simply indicates that the duration of treatment cannot be defined at three-month follow-up after the acute event. In these patients, the option to withdraw anticoagulant treatment should periodically be re-assessed, based on the dynamic balance between the risks of recurrence and bleeding. Lifelong treatment is recommended for most patients with a second unprovoked DVT or PE.
In two recent trials with a total of 1224 patients, extended therapy with aspirin (after termination of standard oral anticoagulation) was associated with a 30–35% reduction in the risk of recurrence after unprovoked DVT and/or PE.368,369 This corresponds to less than half of the risk reduction achieved by oral anticoagulants; on the other hand, the bleeding rates associated with aspirin were low (Table 13).
Clinical trials on extended treatment of venous thromboembolism
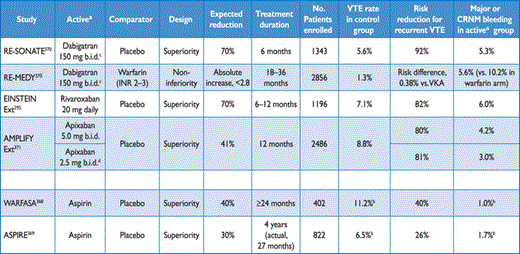 |
 |
b.i.d. = bis in die (twice daily); CRNM = clinically relevant non-major; SD = standard deviation; VKA = vitamin K antagonists; VTE = venous thromboembolism.
a‘Active’ denotes in the Table the direct oral thrombin or factor Xa inhibitor (or aspirin) tested; the comparator arm also received anticoagulation (a vitamin K antagonist) in some of the studies.
bIncidence per patient-year.
cApproved doses of dabigatran are 150 mg b.i.d. and 110 mg b.i.d.
dThis is the approved dose of apixaban for extended treatment.
Clinical trials on extended treatment of venous thromboembolism
 |
 |
b.i.d. = bis in die (twice daily); CRNM = clinically relevant non-major; SD = standard deviation; VKA = vitamin K antagonists; VTE = venous thromboembolism.
a‘Active’ denotes in the Table the direct oral thrombin or factor Xa inhibitor (or aspirin) tested; the comparator arm also received anticoagulation (a vitamin K antagonist) in some of the studies.
bIncidence per patient-year.
cApproved doses of dabigatran are 150 mg b.i.d. and 110 mg b.i.d.
dThis is the approved dose of apixaban for extended treatment.
6.1 New oral anticoagulants for extended treatment
Three NOACs have been evaluated in the extended treatment of patients with VTE: dabigatran, rivaroxaban, and apixaban. In all studies, patients with PE made up approximately one-third of the entire study population, while the remaining two-thirds were patients with DVT but no clinically overt PE. To be included in the extended studies, patients needed to have completed the initial and long-term anticoagulation phase.
The design and principal findings of the recent trials on extended anticoagulation are summarized in Table 13. Dabigatran was compared with warfarin or placebo in two different studies. In RE-MEDY, 2866 patients were randomized to receive dabigatran 150 mg twice daily, or warfarin (INR 2–3). Dabigatran was non-inferior to warfarin for the prevention of confirmed recurrent symptomatic VTE or VTE-related death (HR 1.44; 95% CI 0.78-2.64; P = 0.01 for non-inferiority).370 The rate of major bleeding was 0.9% under dabigatran, compared with 1.8% under warfarin (HR 0.52; 95% CI 0.27–1.02). In the RE-SONATE study, 1353 patients were randomized to dabigatran or placebo for an additional anticoagulation period of 6 months.370) Dabigatran was associated with a 92% risk reduction in symptomatic recurrent VTE or unexplained death (HR 0.08; 95% CI 0.02–0.25). A 0.3% rate of major bleeding was observed in the dabigatran group vs. 0% in the placebo group; major or CRNM bleeding occurred in 5.3% and 1.8% of the patients, respectively (HR 2.92; 95% CI 1.52–5.60).370
The randomized, double-blind EINSTEIN Extension study assessed the efficacy and safety of rivaroxaban for extended treatment of VTE.295 An additional 6- or 12-month course of rivaroxaban (20 mg once daily) was compared with placebo in patients who had completed 6–12 months of anticoagulation treatment for a first VTE. Rivaroxaban had superior efficacy over placebo for preventing recurrent VTE (1.3% vs. 7.1%; HR 0.18; 95% CI 0.09–0.39). Non-fatal major bleeding occurred in 0.7% of patients in the rivaroxaban arm vs. none in the placebo arm. The incidence of major or CRNM bleeding was 6.0% in the rivaroxaban group and 1.2% in the placebo group (HR 5.19; 95% CI 2.3–11.7).
The AMPLIFY Extension study was a double-blind trial in which patients with VTE were randomized to receive two different doses of apixaban (2.5 mg or 5 mg twice daily) or placebo.371 Patients were eligible for inclusion if there was clinical equipoise regarding the continuation or cessation of anticoagulation therapy. The study treatment was given for a 12-month period. Symptomatic recurrent VTE or death from any cause occurred in 11.6% of patients receiving placebo, compared with 3.8% of those receiving 2.5 mg of apixaban (HR 0.33 vs. placebo; 95% CI 0.22–0.48) and with 4.2% of the patients who were receiving 5 mg of apixaban (HR 0.36 vs. placebo; 95% CI 0.25–0.53). The rates of major bleeding were 0.5% in the placebo group, 0.2% in the 2.5 mg apixaban group, and 0.1% in the 5 mg apixaban group; major or CRNM bleeding occurred in 2.7%, 3.2% (HR 1.20 vs. placebo; 95% CI 0.69–2.10) and 4.3% (HR 1.62 vs. placebo; 95% CI 0.96–2.73) of the patients, respectively.
In summary, the results of the trials using NOACs in the extended treatment of VTE are in line with those of the studies that tested these agents in the acute-phase treatment and standard duration of anticoagulation after PE or VTE (discussed in the previous section). They indicate that NOACs are both effective (in terms of prevention of symptomatic or fatal recurrence of VTE) and safe (particularly in terms of major bleeding)—probably safer than standard VKA regimens.
Recommendations for duration of anticoagulation after pulmonary embolism
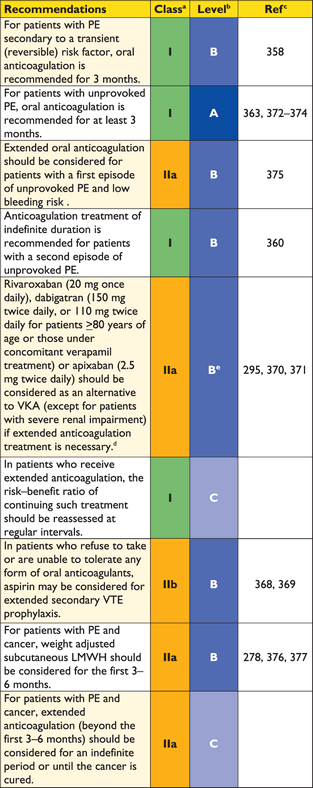 |
 |
LMWH = low molecular weight heparin; PE = pulmonary embolism; VKA = vitamin K antagonist.
aClass of recommendation.
bLevel of evidence.
cReferences.
dLong-term data on patients taking new oral anticoagulants for secondary PE prophylaxis are not yet available.
eB refers to the evidence available for each drug separately.
7. Chronic thromboembolic pulmonary hypertension
7.1 Epidemiology
Chronic thromboembolic pulmonary hypertension is a debilitating disease caused by chronic obstruction of major pulmonary arteries. Although the exact prevalence and annual incidence of CTEPH is unknown, data from the United Kingdom suggest that this condition may occur in approximately five individuals per million population per year.378 According to the 2009 ESC Guidelines on pulmonary hypertension (PH)379 and the recently updated clinical classification of PH,380 CTEPH is listed as a distinct subgroup of PH (group 4).
Chronic thromboembolic pulmonary hypertension has been reported to be a long-term complication of PE, with a reported cumulative incidence of 0.1–9.1% within the first two years after a symptomatic PE event.381 The large margin of error is probably due to referral bias, absence of early symptoms, and the difficulty in differentiating ‘true’ acute PE from an acute episode superimposed on pre-existing CTEPH. Routine screening for CTEPH after PE is not supported by current evidence; a significant number of CTEPH cases develop in the absence of previous acute PE.
7.2 Pathophysiology
The available evidence indicates that CTEPH is primarily caused by pulmonary thromboembolism. In a recent international registry, a clinical history of VTE was recorded in 80% of patients with CTEPH.382 Inadequate anticoagulation, large thrombus mass, residual thrombi, and recurrence of VTE may contribute to the development of CTEPH. On the other hand, CTEPH does not share the same risk factor profile with VTE and has been associated with only a few specific thrombophilic factors. These include the presence of lupus anticoagulant or antiphospholipid antibodies and elevated levels of coagulation factor VIII.4,383 It has been proposed that, in some patients, PE may be followed by a pulmonary vascular remodelling process modified by infection,384 inflammation,385 circulating and vascular-resident progenitor cells,386,387 thyroid hormone replacement, or malignancy.4 Hypercoagulation, ‘sticky’ red blood cells, high platelet counts, and ‘uncleavable’ fibrinogen may further contribute to obliteration of the pulmonary arteries in CTEPH.388 In addition, non-plasmatic risk factors such as splenectomy, ventriculoatrial shunt for hydrocephalus therapy, inflammatory bowel disease, and chronic osteomyelitis are associated with a higher incidence and a worse prognosis of CTEPH.4,389
Apart from major pulmonary vascular obstruction, the pathophysiology of CTEPH includes a pulmonary microvascular disease,390 which may be responsible for the poor outcome in some cases of pulmonary endarterectomy.391 This condition may originate from a high-flow or high-pressure state in previously unaffected vessels, or may be driven by hypoxia, infection, or inflammation.
7.3 Clinical presentation and diagnosis
The median age of patients at diagnosis of CTEPH is 63 years, and both genders are equally affected;392 paediatric cases are rare.393,394 Clinical symptoms and signs are non-specific or absent in early CTEPH, with signs of right heart failure only becoming evident in advanced disease; thus, early diagnosis remains a challenge in CTEPH, with a median time of 14 months between onset of symptoms and diagnosis in expert centres.392 When present, the clinical symptoms of CTEPH may resemble those of acute PE, or of idiopathic pulmonary arterial hypertension (iPAH); in the latter context, oedema and haemoptysis occur more often in CTEPH, while syncope is more common in iPAH.
The diagnosis of CTEPH is based on findings obtained after at least 3 months of effective anticoagulation, in order to discriminate this condition from ‘sub-acute’ PE. These findings are: Some patients, particularly those with complete unilateral obstruction, may present with normal pulmonary haemodynamics at rest, despite symptomatic disease. These patients are also considered as having CTEPH and managed accordingly. Suitable terminology to describe this condition of chronic thromboembolic pulmonary vascular disease is still lacking.
mean pulmonary arterial pressure ≥25 mm Hg, with pulmonary arterial wedge pressure ≤15 mm Hg;
at least one (segmental) perfusion defect detected by perfusion lung scan, or pulmonary artery obstruction seen by MDCT angiography or conventional pulmonary cineangiography.
An algorithm for CTEPH diagnosis is shown in Figure 6. While MDCT angiography is the investigation of choice for the diagnosis of acute PE, planar V/Q lung scan remains the main first-line imaging modality for CTEPH, as it carries a 96–97% sensitivity and 90–95% specificity for the diagnosis.395 By contrast, in iPAH and pulmonary veno-occlusive disease, perfusion scans are either normal or show sub-segmental defects.396
Algorithm for the diagnosis of chronic thromboembolic pulmonary hypertension (adapted from Lang et al. (2010)).397
Right heart catheterization is an essential diagnostic tool. Mean pulmonary artery pressure, pulmonary vascular resistance, and pulmonary arterial wedge pressure are key haemodynamic parameters. In candidates for surgery, pulmonary vascular resistance has prognostic value.398
Multi-detector CT angiography has become an established imaging technique for CTEPH,399 but CT alone cannot rule out the disease.397 CT angiography may help identify complications of the disease, such as pulmonary artery distension, resulting in left main coronary artery compression.
A high-resolution CT scan of the chest delivers images of the lung parenchyma, and identifies emphysema, bronchial disease or interstitial lung disease, as well as infarcts, vascular- and pericardial malformations, and thoracic wall deformities. Perfusion inequalities manifest as a mosaic parenchymal pattern with dark areas corresponding to relatively decreased perfusion. Although a mosaic pattern is frequent in CTEPH, it can also be observed in up to 12% of patients with pulmonary arterial hypertension.400 Magnetic resonance imaging of the pulmonary vasculature is still considered inferior to CT,401 but this modality, as well angioscopy,402 intravascular ultrasound or optical coherence tomography, may be used according to local experience and practice.
The final step in the diagnostic pathway is side-selective pulmonary angiography in the anterior-posterior and lateral projections, illustrating pulmonary artery webs and bands, wall irregularities, stenoses, aneurysms, and complete vascular obstructions, as well as bronchial collaterals.
7.4 Treatment and prognosis
A proposed algorithm for CTEPH treatment is displayed in Figure 7. Pulmonary endarterectomy (PEA) is the treatment of choice for the disease. In Europe, in-hospital mortality is currently as low as 4.7% in expert centres.398 The majority of patients experience substantial relief from symptoms and near-normalization of haemodynamics.391,398,403 In contrast to surgical embolectomy for acute PE, treatment of CTEPH necessitates a true endarterectomy through the medial layer of the pulmonary arteries, which is performed under deep hypothermia and circulatory arrest.404
Algorithm for the treatment of chronic thromboembolic pulmonary hypertension (adapted from Ghofrani et al. (2013)).412
The operability of patients with CTEPH is determined by multiple factors that cannot easily be standardized; these are related to the suitability of the patient, the expertise of the surgical team, and available resources. General criteria include pre-operative New York Heart Association functional class II–IV and the surgical accessibility of thrombi in the main, lobar, or segmental pulmonary arteries. Advanced age per se is no contraindication for surgery. There is no pulmonary vascular resistance threshold or measure of RV dysfunction that absolutely precludes PEA.
Patients who do not undergo surgery, or suffer from persistent or residual pulmonary hypertension after PEA, face a poor prognosis. Advances in balloon pulmonary angioplasty are continuing in an attempt to make this technique a therapeutic alternative for selected patients with non-operable CTEPH.405–408
Optimal medical treatment for CTEPH consists of anticoagulants, diuretics, and oxygen. Lifelong anticoagulation is recommended, even after PEA, while no data exist on the efficacy and safety of new direct oral anticoagulants. Although there is no consensus, routine cava filter placement is not justified by the available evidence. Pulmonary microvascular disease in CTEPH has provided the rationale for use of drugs approved for pulmonary arterial hypertension (PAH).409 These drugs may be justified (i) in inoperable patients, (ii) in patients with persistent or residual pulmonary hypertension after PEA or (iii) in the presence of an unacceptable surgical risk–benefit ratio.
The dual endothelin antagonist bosentan was evaluated over 16 weeks in 157 patients with inoperable CTEPH or persistent/recurrent pulmonary hypertension after PEA; the primary combined endpoint of a decrease in pulmonary vascular resistance (PVR) and an increase in the 6-minute walking distance was not met.410 PVR is defined as mean pulmonary arterial pressure minus pulmonary artery wedge pressure, divided by cardiac output. Riociguat, a soluble, oral stimulator of guanylate cyclase, was administered to 261 of 446 screened patients with inoperable CTEPH—or persistent/recurrent pulmonary hypertension after PEA—for 16 weeks, and led to a mean increase of 39 metres in the 6-minute walking distance (P < 0.001; primary endpoint) and to a least-squared mean difference of 246 dyn.cm.s−5 in pulmonary vascular resistance (P < 0.001; secondary endpoint); the time to clinical worsening remained unchanged.411 Riociguat has received approval for use in the treatment of adults with persistent or recurrent CTEPH after surgical treatment, or inoperable CTEPH, to improve exercise capacity and WHO functional class. Off-label use of drugs approved for PAH, or the use of riociguat as a therapeutic bridge to PEA in patients considered to be at high risk due to poor haemodynamics, is currently not justified.
Recommendations for chronic thomboembolic pulmonary hypertension
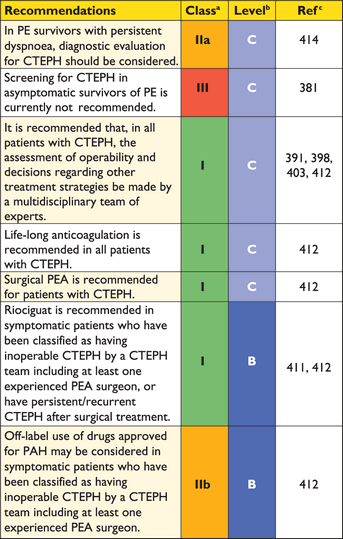 |
 |
CTEPH = chronic thomboembolic pulmonary hypertension; PE = pulmonary embolism; PEA = pulmonary endarterectomy.
aClass of recommendation.
bLevel of evidence.
cReferences.
8. Specific problems
8.1 Pregnancy
Pulmonary embolism is the leading cause of pregnancy-related maternal death in developed countries.415 The risk of PE is higher in the post-partum period, particularly after a caesarean section. Recommendations for the management of venous thromboembolism in pregnancy are included in the 2011 ESC Guidelines on the management of cardiovascular diseases during pregnancy.416 The present section is in agreement with those guidelines.
Pregnancy does not alter the clinical features of PE but, as pregnant women often complain of breathlessness, this symptom should be interpreted with caution. Arterial blood should be drawn with the patient in the upright position, as the partial pressure of oxygen may be lower in the supine position during the third trimester. Data on the validity of clinical prediction rules for PE in pregnancy are lacking, but a recent retrospective series of 125 pregnant women who were referred for CT angiography showed that no patient with an original Wells score of <6 points had a PE.417 These data need to be confirmed in large-scale prospective studies.
8.1.1 Diagnosis of pulmonary embolism in pregnancy
Exposure of the foetus to ionizing radiation is a concern when investigating suspected PE during pregnancy; although this concern is largely overruled by the hazards of missing a potentially fatal diagnosis. This is particularly true for pregnant patients with suspected high-risk PE. Moreover, erroneously assigning a diagnosis of PE to a pregnant woman is also fraught with risks, since it unnecessarily exposes the mother and foetus to the risks of anticoagulant treatment and will impact on delivery plans, future contraception, and thromboprophylaxis during future pregnancies. Therefore, investigations should aim at diagnostic certainty.
The usefulness of D-dimer in pregnancy is controversial. A normal D-dimer value has the same exclusion value for PE in pregnant women as for other patients with suspected PE but is found more rarely, because plasma D-dimer levels physiologically increase throughout pregnancy.127,418 Study of a series of pregnant patients with suspected DVT found that an agglutination assay would have ruled out the disease in 55% of the cases with a negative predictive value of 100%.418 The same study attempted to establish higher cut-off levels in pregnancy for several commonly used D-dimer assays.419 These thresholds await prospective validation and, in the meantime, the usual D-dimer cut-off value should apply to rule out PE in pregnancy. If the D-dimer result is abnormal, diagnostic work-up may continue with lower-limb CUS, since proximal DVT warrants anticoagulation treatment and makes thoracic imaging unnecessary. If ultrasonography is negative, the diagnosis should be pursued.
The amount of radiation absorbed by the foetus during different diagnostic tests is shown in Table 14. The danger threshold for injury to the foetus is considered to be 50 mSv (50 000 µGy),420 and all radiological tests fall well below this figure; nevertheless lung scintigraphy, when available, may be preferred over CT because it avoids the high radiation dose delivered to the female breast by CT angiography and the resulting small but significant increase of the lifetime risk of breast cancer.421 As a rule, a ventilation lung scan is unnecessary as the chest X-ray is usually normal, thus further limiting radiation exposure. The diagnostic yield of scintigraphy is around 80%, with 70% of tests yielding normal perfusion scans and 5–10% yielding high-probability scans.422–428 This is at least as high as that of CT in this particular population, due to a higher proportion of inconclusive CT scans during pregnancy.425 A normal perfusion scan and a negative CT are equally safe for ruling out PE in pregnancy, as shown by several retrospective series.427,429
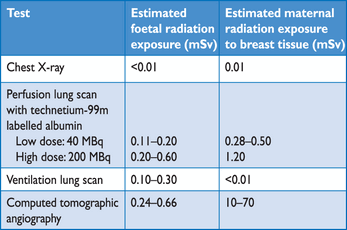 |
 |
mSv = milisievert; PE = pulmonary embolism
 |
 |
mSv = milisievert; PE = pulmonary embolism
Conventional pulmonary angiography carries a significantly higher radiation exposure for the foetus (2.2–3.7 mSv) and should be avoided during pregnancy.420
8.1.2 Treatment of pulmonary embolism in pregnancy
The treatment of PE in pregnancy is based on heparin anticoagulation, because heparin does not cross the placenta and is not found in breast milk in significant amounts. Increasing experience suggests that LMWHs are safe in pregnancy,432–435 and their use is endorsed in several reports.436,437 Treatment should consist of a weight-adjusted dose of LMWH. Adaptation according to anti-Xa monitoring may be considered in women at extremes of body weight or with renal disease, but routine monitoring is generally not justified.279,436,437 Unfractionated heparin is not contraindicated in pregnancy, although it requires aPTT monitoring and is probably more likely to cause osteoporosis if used for longer periods. Fondaparinux should not be used in pregnancy due to the lack of data. VKAs cross the placenta and are associated with a well-defined embryopathy during the first trimester. Administration of VKAs in the third trimester can result in foetal and neonatal haemorrhage, as well as placental abruption. Warfarin may be associated with central nervous system anomalies throughout pregnancy. New oral anticoagulants are contraindicated in pregnant patients.
The management of labour and delivery require particular attention. Epidural analgesia cannot be used unless LMWH has been discontinued at least 12 hours before delivery. Treatment can be resumed 12–24 hours after removal of the epidural catheter. Close collaboration between the obstetrician, the anaesthesiologist, and the attending physician is recommended.
After delivery, heparin treatment may be replaced by anticoagulation with VKA. Anticoagulant treatment should be administered for at least 6 weeks after delivery and with a minimum overall treatment duration of 3 months. VKAs can be given to breast-feeding mothers.
Published data on 28 pregnant women treated with thrombolytic agents—mainly with rtPA at the dose of 100 mg over 2 hours—suggest that the risk of complications for the mother may be similar to that in the non-pregnant population.438 Thrombolytic treatment should not be used peripartum, except for critical cases.
Recommendations for pulmonary embolism in pregnancy
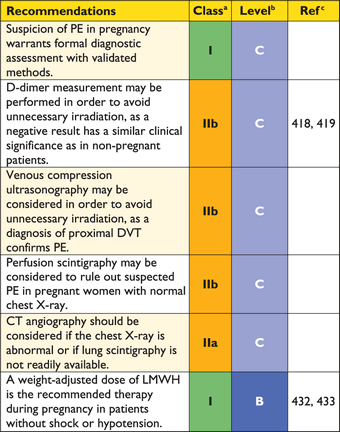 |
 |
CT = computed tomographic; DVT = deep vein thrombosis; LMWH = low molecular weight heparin; PE = pulmonary embolism.
aClass of recommendation.
bLevel of evidence.
cReferences.
8.2 Pulmonary embolism and cancer
The overall risk of venous thromboembolism in cancer patients is four times as great as in the general population.8 Although the largest absolute numbers of VTE episodes occur in patients with lung, colon, and prostate cancer, the relative risk for VTE is highest in multiple myeloma, brain, and pancreatic cancer (46-, 20-, and 16-fold increased vs. healthy controls, respectively).439 In the metastatic stage, stomach, bladder, uterine, renal, and lung cancer are also associated with a high incidence of VTE.17
Patients receiving chemotherapy have a six-fold increase in the adjusted risk ratio for VTE compared with a healthy population.8 Nevertheless, prophylactic anticoagulation is not routinely recommended during ambulatory anti-cancer chemotherapy, with the exception of thalidomide- or lenalidomide-based regimens in multiple myeloma.440,441 LMWH or VKA are not effective in preventing thrombosis related to the use of permanent central venous lines in cancer patients.441
The risk of VTE increases over 90-fold in the first 6 weeks after cancer surgery, compared with that in healthy controls, and is second only to the risk of VTE after hip or knee replacement surgery. Notably, the risk of VTE after cancer surgery remains elevated (up to 30-fold) between the fourth and twelfth post-operative month.442 Continued vigilance is therefore necessary, as currently recommended prophylactic anticoagulation covers only the first 30 days after cancer surgery.
8.2.1 Diagnosis of pulmonary embolism in patients with cancer
Malignancy is taken into account in the estimation of clinical probability of PE (see Section 3). A negative D-dimer test has the same diagnostic value as in non-cancer patients. On the other hand, D-dimer levels are non-specifically increased in many patients with cancer. In one study, raising the D-dimer cut-off level to 700 µg/L, or using age-dependent cut-off levels, increased the proportion of cancer patients in whom PE could be ruled out from 8.4 to 13% and 12%, respectively; the corresponding false-negative rates appeared acceptable.443 This strategy needs further validation.
The widespread use of CT scanners has resulted in an increasing number of incidentally diagnosed, asymptomatic PEs in cancer patients.444 Their significance, particularly if limited to segmental or sub-segmental arteries, is unclear; however, in view of the high risk of an adverse outcome reported by uncontrolled studies,445–449 the treatment strategies recommended for symptomatic PE should be also considered for incidental PE found in patients with malignancy.
8.2.2 Prognosis for pulmonary embolism in patients with cancer
Cancer is a risk factor for an adverse outcome in acute PE. In a multivariate analysis of 570 patients with PE, the presence of cancer tripled the 30-day risk of death, shock, or recurrence of PE.257 In the RIETE registry, in patients with and without cancer, all-cause three-month mortality were 26.4% and 4.1% respectively (P < 0.001). Among over 35 000 patients with VTE, cancer was the strongest independent risk factor for both all-cause and PE-related mortality.20 The worse outcome is due to the increased bleeding risk during anticoagulation therapy and to the high rate of recurrence of VTE.450–454
The risk of recurrence of PE in cancer was recently assessed in a cohort study of 543 patients and was validated in an independent set of 819 patients.453 A suggested score predicting risk of recurrence included breast cancer (minus 1 point), tumour node metastasis Stage I or II (minus 1 point), female sex, lung cancer, and previous VTE (plus 1 point each). Patients with a score ≤0 were at low risk (≤4.5%) and those with a score >1 were at high risk (≥19%) of recurrence of VTE.453 The score may assist in making individually tailored decisions regarding the duration of anticoagulant treatment.
8.2.3 Management of pulmonary embolism in patients with cancer
When selecting the mode of anticoagulation in patients with cancer and acute PE, LMWH administered in the acute phase (except for high-risk PE) and continued over the first 3–6 months should be considered as first-line therapy. However, this strategy is based largely on the results of a single trial with an observed 50% reduction in the rate of recurrence of VTE without increased bleeding risk, as compared with the early transition from heparin to VKA.376,377 Evidence regarding treatment of cancer-related PE with fondaparinux and the new oral anticoagulants is limited.
Chronic anticoagulation may consist of continuation of LMWH, transition to VKA, or discontinuation of anticoagulation. The decisions should be made on a case-by-case basis after considering the success of anti-cancer therapy, the estimated risk of recurrence of VTE, the bleeding risk, and the preference of the patient. Periodic reassessment of the risk–benefit ratio of chronic anticoagulant treatment is a reasonable strategy.
Recurrence of VTE in cancer patients on VKA or LMWH therapy may be managed by changing to the highest permitted dose of LMWH or opting for vena cava filter placement.455 Venous filters should primarily be considered when anticoagulation is impossible due to haemorrhage; however, the risk of filter thrombosis in the absence of anticoagulation may be particularly high in cancer patients. In a recent prospective, randomized trial in cancer patients with DVT or PE, no clinical advantage was found in placement of a vena cava filter in addition to anticoagulation with fondaparinux.456
8.2.4 Occult cancer presenting as unprovoked pulmonary embolism
Approximately 10% of patients presenting with unprovoked PE will develop cancer within the next 5–10 years, with the majority of cases appearing in the first 1–2 years after diagnosis of PE.457 Recently, Sorensen et al. reported that cancer will emerge with a similarly high incidence after unprovoked VTE and after that provoked by surgery, but more often than after post-traumatic VTE.458 The evidence supporting screening for occult cancer after unprovoked VTE is inconclusive. Di Nisio et al. recommended, as the most effective and least harmful approach for such patients, a screening strategy of performing pelvic and abdominal CT combined with mammography and sputum cytology.459 However, when such an extensive screening strategy was compared with basic clinical evaluation, no benefit was found in terms of 5-year survival.460 Therefore, the search for occult cancer after an episode of VTE may be restricted to careful history, physical examination, basic laboratory tests, and a chest X-ray.461,462
Recommendations for pulmonary embolism in cancer
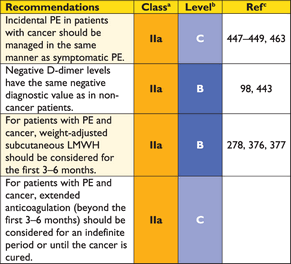 |
 |
LMWH = low molecular weight heparin; PE = pulmonary embolism.
aClass of recommendation.
bLevel of evidence.
cReferences.
8.3 Non-thrombotic pulmonary embolism
Different cell types can cause non-thrombotic embolization, including adipocytes, haematopoietic, amniotic, trophoblastic, and tumour cells. In addition, bacteria, fungi, parasites, foreign materials, and gas can lead to PE. Symptoms are similar to those of acute VTE and include dyspnoea, tachycardia, chest pain, cough, and occasionally haemoptysis, cyanosis, and syncope.
Diagnosis of non-thrombotic PE can be a challenge.464 In the case of small particles, microemboli cannot be detected on CT images. An overview of typical imaging findings for the various types of non-thrombotic PE has been provided.465 Given the rarity of this disease, clinical evidence is limited and mainly based on small case series.
8.3.1 Septic embolism
Septic embolism to the pulmonary circulation is a relatively rare clinical event and is commonly associated with right-sided endocarditis. Risk factors include intravenous drug abuse and infected indwelling catheters or pacemaker wires. Other causes include septic thrombophlebitis from the tonsils and the jugular, dental, and pelvic regions. The diagnosis is based on identifying the source of septic emboli, positive blood culture tests, and chest X-ray or CT after considering the clinical context. Although Staphylococcus aureus is the most common bacterial pathogen, the increasing number of immunocompromised patients—and those with indwelling catheters and vascular prostheses—leads to a rise in the incidence of anaerobic gram positive and -negative bacteria, bacterioide species, and fungi.466 Specific treatment of the responsible bacterial or fungal micro-organism is necessary.
8.3.2 Foreign-material pulmonary embolism
The increasing use of interventional techniques in modern medicine has drastically increased the incidence of foreign-material PE.467 Examples of foreign material include silicone, broken catheters, guide wires, vena cava filters, coils for embolization, and endovascular stent components. If possible, intravascular foreign objects should be removed, since the material may cause further thrombosis and sepsis.
8.3.3 Fat embolism
Embolization of fat occurs in almost all patients with pelvic or long-bone fractures and in those undergoing endomedullary nailing or placement of knee and hip prostheses, but also during lipid and propofol infusion, intra-osseous infusion and bone marrow harvest, and in sickle cell disease, fatty liver disease, pancreatitis, and after liposuction. Pulmonary involvement is not only due to vascular obstruction but also to the release of substances triggering an inflammatory cascade, thus explaining why some patients with fat embolism develop acute respiratory distress syndrome.468
The classical triad of fat embolization is characterized by altered mental status, respiratory distress, and petechial rash occurring typically 12–36 hours after injury. Fat globules can be found in blood, urine, sputum, broncho-alveolar lavage, and cerebrospinal fluid.469 In most cases the condition is self-limiting. Treatment should be supportive. Although the successful use of high doses of methyl prednisolone has been reported in humans, along with the positive effects of phorbol myristate acetate and sivelestat in animals, there is no evidence that these drugs alter the course of the disease.470
8.3.4 Air embolism
Although air embolism can occur in both the venous and arterial systems, venous emboli are more common. Venous air embolization is often an iatrogenic complication of the manipulation of central venous and haemodialysis catheters. The lethal volume of air after injection in humans is estimated to range from 100 to 500 mL.471 The major effect of venous air embolism is the obstruction of the right ventricular outflow tract, or of the pulmonary arterioles, by a mixture of air bubbles and fibrin. Although the diagnosis can be made by X-ray or echocardiography, CT scanning may be the most sensitive diagnostic test, showing a unique picture of round or mirror-shaped densities localized ventrally in the supine patient.465 Treatment includes maintenance of the circulation, prevention of further entry of gas, and volume expansion. The patient should be placed in the left lateral decubitus position to prevent right ventricular outflow obstruction by airlock.472 In the case of large amounts of central air, aspiration with the use of a central venous catheter might be an option. Administration of up to 100% oxygen can decrease bubble size by establishing a diffusion gradient that favours elimination of the gas.471
8.3.5 Amniotic fluid embolism
Amniotic fluid embolism is a rare but catastrophic complication unique to pregnancy. Estimated incidences, obtained through validated case identification, range between 1.9 and 2.5 cases per 100 000 maternities.473 The most likely mechanism is that amniotic fluid is forced into the uterine veins during normal labour or when the placenta is disrupted by surgery or trauma. As a consequence, pulmonary vessels are obstructed by cell groups and meconium, and an inflammatory reaction occurs due to the release of active metabolites. The majority of patients develop seizures. Some patients are diagnosed with pulmonary oedema and acute respiratory distress syndrome later in the course of the event. Mortality is high—up to 21%, even in recent cohort studies.473 Management should be supportive.
8.3.6 Tumour embolism
Pulmonary intravascular tumour emboli are seen in up to 26% of autopsies of patients with solid malignancies, although the diagnosis is rarely made before death.474 Carcinoma of the prostate gland, digestive system, liver, and breast is most commonly implicated. Radiologically, tumour microembolism may mimic many lung conditions, including pneumonia, tuberculosis, and interstitial lung disease, whereas tumour macroembolism is indistinguishable from VTE. Treatment should target the underlying malignant disease.
9. Appendix
ESC National Cardiac Societies actively involved in the review process of the 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism.
Austria, Austrian Society of Cardiology, Nika Skoro-Sajer – Azerbaijan, Azerbaijan Society of Cardiology, Ruslan Najafov – Belarus, Belorussian Scientific Society of Cardiologists, Svetlana Sudzhaeva – Belgium, Belgian Society of Cardiology, Michel De Pauw – Bosnia and Herzegovina, Association of Cardiologists of Bosnia & Herzegovina, Fahir Baraković – Bulgaria, Bulgarian Society of Cardiology, Mariya Tokmakova – Croatia, Croatian Cardiac Society, Bosko Skoric – Czech Republic, Czech Society of Cardiology, Richard Rokyta – Denmark, Danish Society of Cardiology, Morten Lock Hansen – Estonia, Estonian Society of Cardiology, Märt Elmet – Finland, Finnish Cardiac Society, Veli-Pekka Harjola – France, French Society of Cardiology, Guy Meyer – Georgia, Georgian Society of Cardiology, Archil Chukhrukidze – Germany, German Cardiac Society, Stephan Rosenkranz – Greece, Hellenic Cardiological Society, Aristides Androulakis – Hungary, Hungarian Society of Cardiology, Tamás Forster – Italy, Italian Federation of Cardiology, Francesco Fedele – Kyrgyzstan, Kyrgyz Society of Cardiology, Talant Sooronbaev – Latvia, Latvian Society of Cardiology, Aija Maca – Lithuania, Lithuanian Society of Cardiology, Egle Ereminiene – Malta, Maltese Cardiac Society, Josef Micallef – Norway, Norwegian Society of Cardiology, Arne Andreasen – Poland, Polish Cardiac Society, Marcin Kurzyna – Portugal, Portuguese Society of Cardiology, Daniel Ferreira – Romania, Romanian Society of Cardiology, Antoniu Octavian Petris – Russia, Russian Society of Cardiology, Sergey Dzemeshkevich – Serbia, Cardiology Society of Serbia, Milika Asanin – Slovakia, Slovak Society of Cardiology, Iveta Šimkova – Spain, Spanish Society of Cardiology, Manuel Anguita – Sweden, Swedish Society of Cardiology, Christina Christersson – The Former Yugoslav Republic of Macedonia, Macedonian FYR Society of Cardiology, Nela Kostova – Tunisia, Tunisian Society of Cardiology and Cardio-Vascular Surgery, Hedi Baccar – Turkey, Turkish Society of Cardiology, Leyla Elif Sade – Ukraine, Ukrainian Association of Cardiology, Alexander Parkhomenko – United Kingdom, British Cardiovascular Society, Joanna Pepke-Zaba.
References
Author notes
ESC Committee for Practice Guidelines (CPG): Jose Luis Zamorano (Chairperson) (Spain), Stephan Achenbach (Germany), Helmut Baumgartner (Germany), Jeroen J. Bax (Netherlands), Hector Bueno (Spain), Veronica Dean (France), Christi Deaton (UK), Çetin Erol (Turkey), Robert Fagard (Belgium), Roberto Ferrari (Italy), David Hasdai (Israel), Arno Hoes (Netherlands), Paulus Kirchhof (Germany/UK), Juhani Knuuti (Finland), Philippe Kolh (Belgium), Patrizio Lancellotti (Belgium), Ales Linhart (Czech Republic), Petros Nihoyannopoulos (UK), Massimo F. Piepoli (Italy), Piotr Ponikowski (Poland), Per Anton Sirnes (Norway), Juan Luis Tamargo (Spain), Michal Tendera (Poland), Adam Torbicki (Poland), William Wijns (Belgium), Stephan Windecker (Switzerland).
Document Reviewers: Çetin Erol (CPG Review Coordinator) (Turkey), David Jimenez (Review Coordinator) (Spain), Walter Ageno (Italy), Stefan Agewall (Norway), Riccardo Asteggiano (Italy), Rupert Bauersachs (Germany), Cecilia Becattini (Italy), Henri Bounameaux (Switzerland), Harry R. Bü ller (Netherlands), Constantinos H. Davos (Greece), Christi Deaton (UK), Geert-Jan Geersing (Netherlands), Miguel Angel Gómez Sanchez (Spain), Jeroen Hendriks (Netherlands), Arno Hoes (Netherlands), Mustafa Kilickap (Turkey), Viacheslav Mareev (Russia), Manuel Monreal (Spain), Joao Morais (Portugal), Petros Nihoyannopoulos (UK), Bogdan A. Popescu (Romania), Olivier Sanchez† (France), Alex C. Spyropoulos (USA).
The disclosure forms provided by the experts involved in the development of these guidelines are available on the ESC website www.escardio.org/guidelines.
Representing the European Respiratory Society
Other ESC entities having participated in the development of this document:
ESC Associations: Acute Cardiovascular Care Association (ACCA), European Association for Cardiovascular Prevention & Rehabilitation (EACPR), European Association of Cardiovascular Imaging (EACVI), Heart Failure Association (HFA), ESC Councils: Council on Cardiovascular Nursing and Allied Professions (CCNAP), Council for Cardiology Practice (CCP), Council on Cardiovascular Primary Care (CCPC)
ESC Working Groups: Cardiovascular Pharmacology and Drug Therapy, Nuclear Cardiology and Cardiac Computed Tomography, Peripheral Circulation, Pulmonary Circulation and Right Ventricular Function, Thrombosis.
Disclaimer: The ESC Guidelines represent the views of the ESC and were produced after careful consideration of the scientific and medical knowledge and the evidence available at the time of their publication.
The ESC is not responsible in the event of any contradiction, discrepancy and/or ambiguity between the ESC Guidelines and any other official recommendations or guidelines issued by the relevant public health authorities, in particular in relation to good use of healthcare or therapeutic strategies. Health professionals are encouraged to take the ESC Guidelines fully into account when exercising their clinical judgment, as well as in the determination and the implementation of preventive, diagnostic or therapeutic medical strategies; however, the ESC Guidelines do not override, in any way whatsoever, the individual responsibility of health professionals to make appropriate and accurate decisions in consideration of each patient's health condition and in consultation with that patient and, where appropriate and/or necessary, the patient's caregiver. Nor do the ESC Guidelines exempt health professionals from taking into full and careful consideration the relevant official updated recommendations or guidelines issued by the competent public health authorities, in order to manage each patient's case in light of the scientifically accepted data pursuant to their respective ethical and professional obligations. It is also the health professional's responsibility to verify the applicable rules and regulations relating to drugs and medical devices at the time of prescription.
National Cardiac Societies document reviewers: listed in the Appendix.



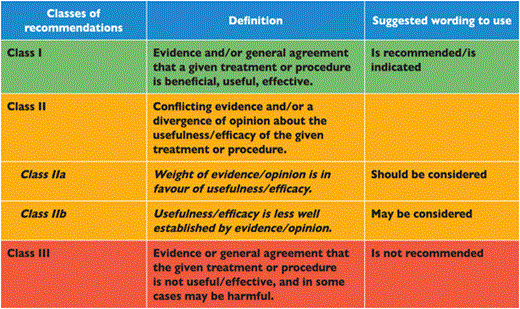
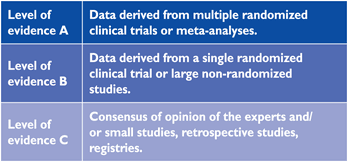
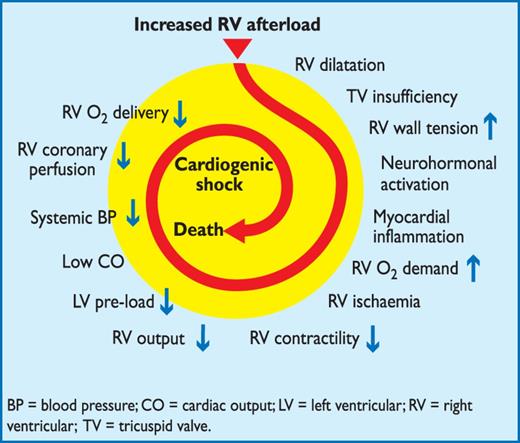
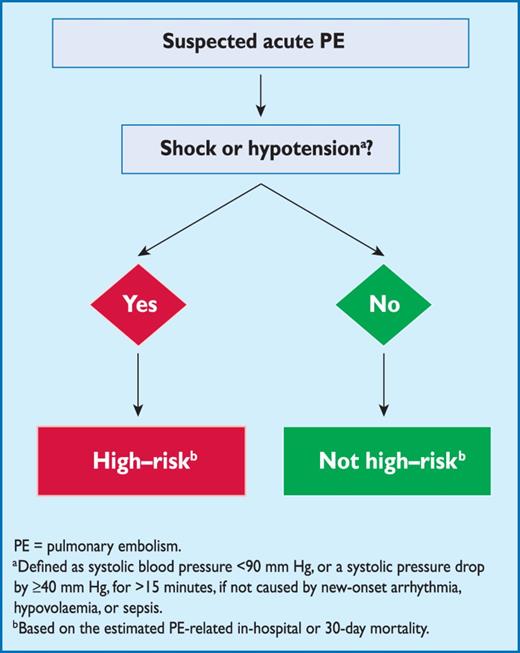
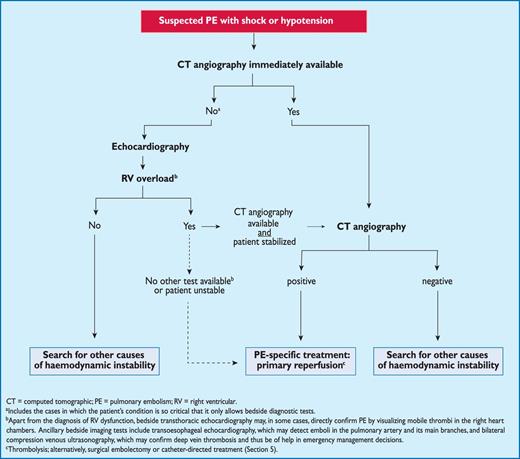
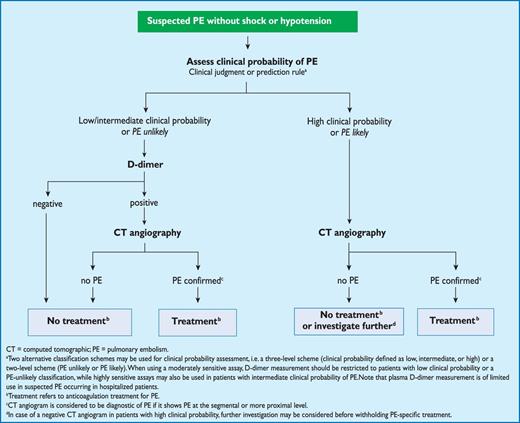
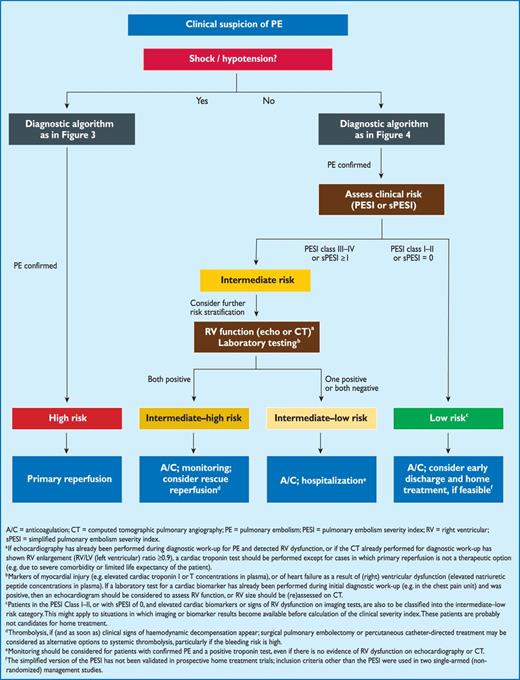
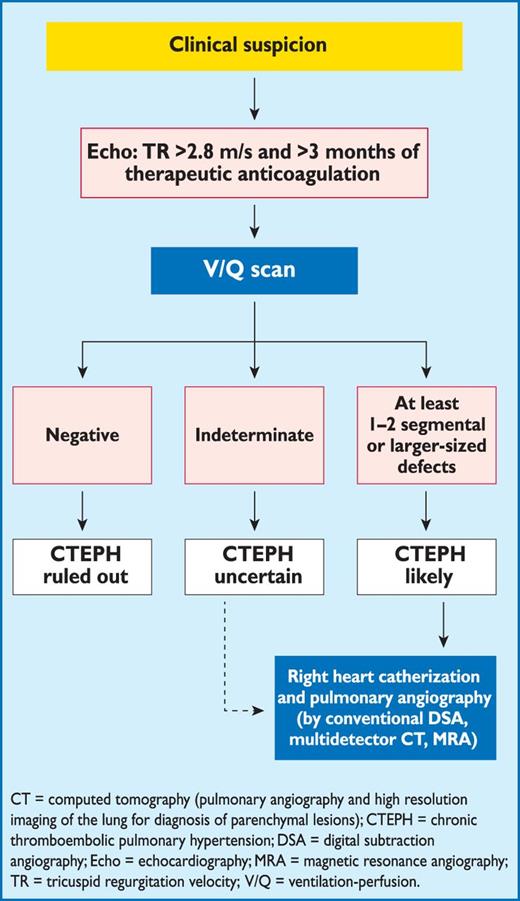
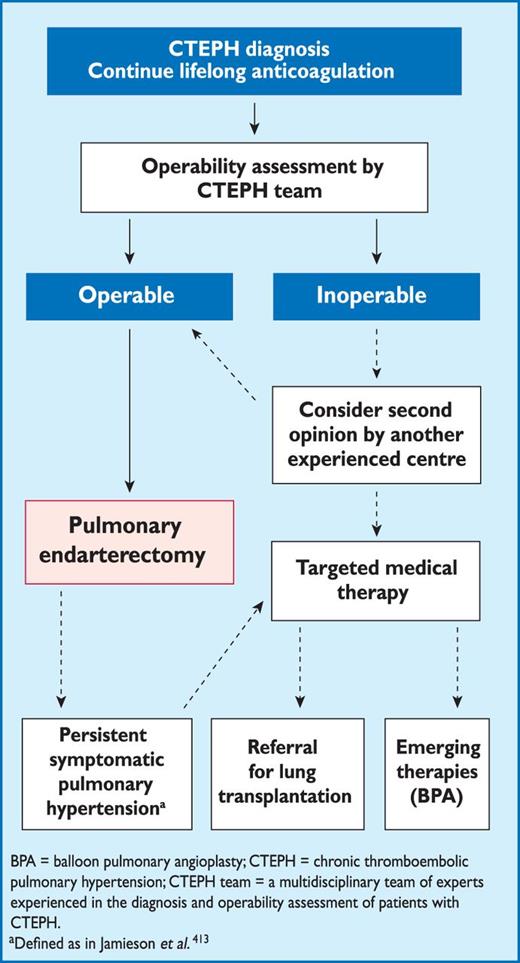

Comments
Initial IV loading dose of 30 mg enoxaparin was recommended for the treatment of ACS because it allows to immediately reaching therapeutic and steady-state conditions, and it's safe and efficacious (1, 2). In the acute phase of PE, similar emergency situation as ACS, IV enoxaparin treatment was not mentioned by current guidelines (3). In patients with acute PE, anticoagulation is objectively preventing both early death and recurrent symptomatic or fatal VET (3). In our opinion, therapeutic anticoagulation with IV enoxaparin seems to be more efficacious for patients in whom primary reperfusion is not considered, and could be a reasonable treatment option for patients in whom primary reperfusion is considered.
Literature
1.Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, Liebson PR, Mukherjee D, Peterson ED, Sabatine MS, Smalling RW, Zieman SJ. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014 Sep 18. pii: S0735-1097(14)06279-2. doi: 10.1016/j.jacc.2014.09.017.
2. Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC), Steg PG, James SK, Atar D, Badano LP, Bl?mstrom-Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez-Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, van 't Hof A, Widimsky P, Zahger D. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012 Oct;33(20):2569-619. doi: 10.1093/eurheartj/ehs215. Epub 2012 Aug 24.
3. Authors/Task Force Members, Konstantinides S, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Gali? N, Gibbs JSR, Huisman M, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack C, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, Svitil P, Noordegraaf AV, Zamorano JL, Zompatori M, Zamorano JL, Torbicki A, Erol C, Deaton C, Hoes A, et al. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism: The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC)Endorsed by the European Respiratory Society (ERS). European Heart Journal. 2014.
Conflict of Interest:
None declared
The cumulative incidence of chronic thromboembolic pulmonary hypertension (CTEPH) as long-term sequela after acute pulmonary embolism is not exactly known. Several studies have reported a rate of 1-2% (1,2), which might be underreported due to the fact that patients claim non- specific symptoms and the awareness for the disease is still low. Early diagnosis of CTEPH thus is challenging. The diagnostic model proposed by Klok and coworkers (3), based on evaluation of three ECG parameters and measurement of NT-proBNP seems to provide an excellent negative predictive value, even if the incidence of CTEPH would be underestimated. The use of this algorithm may avoid complications and costs associated with unnecessary tests. In our opinion, this elegant and inexpensive approach for definitive exclusion of CTEPH deserves being mentioned in the current guidelines (4).
Mattia Arrigo (1) Lars C. Huber (2)
1) University Heart Center, University Hospital Zurich, Switzerland 2) Division of Pulmonology, University Hospital Zurich, Switzerland
Literature
1. Pengo V, Lensing AWA, Prins MH, Marchiori A, Davidson BL, Tiozzo F, Albanese P, Biasiolo A, Pegoraro C, Iliceto S, Prandoni P, Thromboembolic Pulmonary Hypertension Study Group. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350:2257-2264. 2. Becattini C, Agnelli G, Pesavento R, Silingardi M, Poggio R, Taliani MR, Ageno W. Incidence of chronic thromboembolic pulmonary hypertension after a first episode of pulmonary embolism. Chest. 2006;130:172-175. 3. Klok FA, Surie S, Kempf T, Eikenboom J, van Straalen JP, van Kralingen KW, van Dijk APJ, Vliegen HW, Bresser P, Wollert KC, Huisman MV. A simple non-invasive diagnostic algorithm for ruling out chronic thromboembolic pulmonary hypertension in patients after acute pulmonary embolism. Thromb Res. 2011;128:21-26. 4. Authors/Task Force Members, Konstantinides S, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Gali? N, Gibbs JSR, Huisman M, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack C, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, Svitil P, Noordegraaf AV, Zamorano JL, Zompatori M, Zamorano JL, Torbicki A, Erol C, Deaton C, Hoes A, et al. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism: The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC)Endorsed by the European Respiratory Society (ERS). European Heart Journal. 2014.
Conflict of Interest:
None declared