Key Points
-
Tuberculosis remains a serious global health problem and a lack of suitable biomarkers is holding back the evaluation of new tuberculosis vaccine candidates, the improvement of diagnostics and the development of more effective and shorter treatment regimens.
-
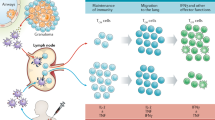
The stages of host–pathogen interactions include: an innate immune phase, during which Mycobacterium tuberculosis may be cleared without the sensitization of B or T cells; an adaptive immune phase in which immunological sensitization does take place; a quiescent infection phase during which the immune system prevents replication of M. tuberculosis in granulomas but fails to eradicate the organism; and a replicating phase, which can lead to clinical disease and the uncontrolled dissemination of bacteria.
-
Correlates of protection against tuberculosis and correlates of risk for tuberculosis will facilitate screening of new vaccine candidates, but there is currently a lack of such correlates. Several immune responses have been recognized as crucial for protection against tuberculosis, including interferon-γ production, CD4+ T cell responses (involving an optimal balance between TH1, TH2, TH17 and TReg cells) and polyfunctional T cell responses. However, these responses are not sufficient for protection and do not therefore represent correlates of risk or protection.
-
Combinations of host molecules, including cytokines, acute-phase proteins, proteins released during tissue damage and serological markers, may constitute diagnostic tests for active tuberculosis in the future.
-
Host immunological markers measured pretreatment, during early treatment and during the final months of treatment reflect bacterial burden and the level of inflammation and may aid new drug development and the clinical management of individual patients.
-
The lack of suitable biomarkers for tuberculosis suggests that appropriate markers should be sought through unbiased 'omics' approaches, followed by thorough hypothesis-driven investigations to develop qualified biomarkers.
Abstract
Currently there are no sufficiently validated biomarkers to aid the evaluation of new tuberculosis vaccine candidates, the improvement of tuberculosis diagnostics or the development of more effective and shorter treatment regimens. To date, the detection of Mycobacterium tuberculosis or its products has not been able to adequately address these needs. Understanding the interplay between the host immune system and M. tuberculosis may provide a platform for the identification of suitable biomarkers, through both unbiased and targeted hypothesis-driven approaches. Here, we review immunological markers, their relation to M. tuberculosis infection stages and their potential use in the fight against tuberculosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
World Health Organisation. Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response. WHO [online], (2010).
Corbett, E. L. et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch. Intern. Med. 163, 1009–1021 (2003).
Abu-Raddad, L. J. et al. Epidemiological benefits of more-effective tuberculosis vaccines, drugs, and diagnostics. Proc. Natl Acad. Sci. USA 106, 13980–13985 (2009).
Davies, P. D. & Pai, M. The diagnosis and misdiagnosis of tuberculosis. Int. J. Tuberc. Lung Dis. 12, 1226–1234 (2008).
Boehme, C. C. et al. Rapid molecular detection of tuberculosis and rifampin resistance. N. Engl. J. Med. 363, 1005–1015 (2010). This paper describes the biggest breakthrough in tuberculosis diagnostics in decades: a direct ex vivo M. tuberculosis gene amplification test for the diagnosis and detection of rifampicin resistance.
Black, G. F. et al. BCG-induced increase in interferon-γ response to mycobacterial antigens and efficacy of BCG vaccination in Malawi and the UK: two randomised controlled studies. Lancet 359, 1393–1401 (2002).
Barry, C. E. et al. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nature Rev. Microbiol. 7, 845–855 (2009).
Sudre, P., ten Dam, G. & Kochi, A. Tuberculosis: a global overview of the situation today. Bull. World Health Organ. 70, 149–159 (1992).
Lawn, S. D. & Churchyard, G. Epidemiology of HIV-associated tuberculosis. Curr. Opin. HIV AIDS 4, 325–333 (2009).
Ottenhoff, T. H., Verreck, F. A., Hoeve, M. A. & van deVosse, E. Control of human host immunity to mycobacteria. Tuberculosis 85, 53–64 (2005).
Lawn, S. D., Myer, L., Edwards, D., Bekker, L. G. & Wood, R. Short-term and long-term risk of tuberculosis associated with CD4 cell recovery during antiretroviral therapy in South Africa. AIDS 23, 1717–1725 (2009).
Khader, S. A. et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nature Immunol. 8, 369–377 (2007).
Vordermeier, H. M. et al. Viral booster vaccines improve Mycobacterium bovis BCG-induced protection against bovine tuberculosis. Infect. Immun. 77, 3364–3373 (2009).
Green, A. M. et al. CD4+ regulatory T cells in a cynomolgus macaque model of Mycobacterium tuberculosis infection. J. Infect. Dis. 202, 533–541 (2010).
Lazar-Molnar, E. et al. Programmed death-1 (PD-1)-deficient mice are extraordinarily sensitive to tuberculosis. Proc. Natl Acad. Sci. USA 107, 13402–13407 (2010).
Bruns, H. et al. Anti-TNF immunotherapy reduces CD8+ T cell-mediated antimicrobial activity against Mycobacterium tuberculosis in humans. J. Clin. Invest. 119, 1167–1177 (2009).
Stenger, S. et al. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science 282, 121–125 (1998).
Cooper, A. M. Cell-mediated immune responses in tuberculosis. Annu. Rev. Immunol. 27, 393–422 (2009).
Gallegos, A. M., Pamer, E. G. & Glickman, M. S. Delayed protection by ESAT-6-specific effector CD4+ T cells after airborne M. tuberculosis infection. J. Exp. Med. 205, 2359–2368 (2008).
Gonzalez-Juarrero, M. et al. Temporal and spatial arrangement of lymphocytes within lung granulomas induced by aerosol infection with Mycobacterium tuberculosis. Infect. Immun. 69, 1722–1728 (2001).
Qin, L., Gilbert, P. B., Corey, L., McElrath, M. J. & Self, S. G. A framework for assessing immunological correlates of protection in vaccine trials. J. Infect. Dis. 196, 1304–1312 (2007).
Comstock, G. W. Field trials of tuberculosis vaccines: how could we have done them better? Control. Clin. Trials 15, 247–276 (1994).
Kagina, B. M. et al. Specific T cell frequency and cytokine expression profile do not correlate with protection against tuberculosis after bacillus Calmette-Guerin vaccination of newborns. Am. J. Respir. Crit. Care Med. 182, 1073–1079 (2010). This study shows that polyfunctional T cells and cytokine expression do not correlate with BCG-induced protection against tuberculosis.
Darrah, P. A. et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nature Med. 13, 843–850 (2007).
Chen, C. Y. et al. A critical role for CD8 T cells in a nonhuman primate model of tuberculosis. PLoS Pathog. 5, e1000392 (2009).
Giri, P. K., Verma, I. & Khuller, G. K. Enhanced immunoprotective potential of Mycobacterium tuberculosis Ag85 complex protein based vaccine against airway Mycobacterium tuberculosis challenge following intranasal administration. FEMS Immunol. Med. Microbiol. 47, 233–241 (2006).
Agger, E. M. et al. Protective immunity to tuberculosis with Ag85B-ESAT-6 in a synthetic cationic adjuvant system IC31. Vaccine 24, 5452–5460 (2006).
Bennekov, T. et al. Alteration of epitope recognition pattern in Ag85B and ESAT-6 has a profound influence on vaccine-induced protection against Mycobacterium tuberculosis. Eur. J. Immunol. 36, 3346–3355 (2006).
Hoft, D. F. et al. Investigation of the relationships between immune-mediated inhibition of mycobacterial growth and other potential surrogate markers of protective Mycobacterium tuberculosis immunity. J. Infect. Dis. 186, 1448–1457 (2002).
Cheon, S. H. et al. Bactericidal activity in whole blood as a potential surrogate marker of immunity after vaccination against tuberculosis. Clin. Diagn. Lab. Immunol. 9, 901–907 (2002).
Spencer, C. T., Abate, G., Blazevic, A. & Hoft, D. F. Only a subset of phosphoantigen-responsive γ9δ2 T cells mediate protective tuberculosis immunity. J. Immunol. 181, 4471–4484 (2008).
Wallis, R. S. et al. Biomarkers and diagnostics for tuberculosis: progress, needs, and translation into practice. Lancet 375, 1920–1937 (2010). This is a comprehensive review of diagnostic and other biomarkers for tuberculosis.
Lalvani, A. et al. Enhanced contact tracing and spatial tracking of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Lancet 357, 2017–2021 (2001).
Mori, T. et al. Specific detection of tuberculosis infection: an interferon-γ-based assay using new antigens. Am. J. Respir. Crit. Care Med. 170, 59–64 (2004).
Ferrara, G. et al. Exploring the immune response against Mycobacterium tuberculosis for a better diagnosis of the infection. Arch. Immunol. Ther. Exp. 57, 425–433 (2009).
Pai, M., Zwerling, A. & Menzies, D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann. Intern. Med. 149, 177–184 (2008).
Mazurek, G. H. et al. Prospective comparison of the tuberculin skin test and 2 whole-blood interferon-γ release assays in persons with suspected tuberculosis. Clin. Infect. Dis. 45, 837–845 (2007).
Janssens, J. P. Interferon-γ release assay tests to rule out active tuberculosis. Eur. Respir. J. 30, 183–184 (2007).
Losi, M. et al. Use of a T-cell interferon-γ release assay for the diagnosis of tuberculous pleurisy. Eur. Respir. J. 30, 1173–1179 (2007).
Thomas, M. M. et al. Rapid diagnosis of Mycobacterium tuberculosis meningitis by enumeration of cerebrospinal fluid antigen-specific T-cells. Int. J. Tuberc. Lung Dis. 12, 651–657 (2008).
Chegou, N. N., Black, G. F., Kidd, M., van Helden, P. D. & Walzl, G. Host markers in QuantiFERON supernatants differentiate active TB from latent TB infection: preliminary report. BMC Pulm. Med. 9, 21 (2009).
Wu, B. et al. Messenger RNA expression of IL-8, FOXP3, and IL-12β differentiates latent tuberculosis infection from disease. J. Immunol. 178, 3688–3694 (2007).
Djoba Siawaya, J. F. et al. Differential cytokine/chemokines and KL-6 profiles in patients with different forms of tuberculosis. Cytokine 47, 132–136 (2009).
Caccamo, N. et al. Multifunctional CD4+ T cells correlate with active Mycobacterium tuberculosis infection. Eur. J. Immunol. 40, 2211–2220 (2010).
Casey, R. et al. Enumeration of functional T-cell subsets by fluorescence-immunospot defines signatures of pathogen burden in tuberculosis. PLoS ONE 5, e15619 (2010).
Harari, A. et al. Dominant TNF-α+Mycobacterium tuberculosis-specific CD4+ T cell responses discriminate between latent infection and active disease. Nature Med. 17, 372–376 (2011).
Steingart, K. R. et al. A systematic review of commercial serological antibody detection tests for the diagnosis of extrapulmonary tuberculosis. Thorax 62, 911–918 (2007).
Steingart, K. R. et al. Performance of purified antigens for serodiagnosis of pulmonary tuberculosis: a meta-analysis. Clin. Vaccine Immunol. 16, 260–276 (2009).
Kunnath-Velayudhan, S. et al. Dynamic antibody responses to the Mycobacterium tuberculosis proteome. Proc. Natl Acad. Sci. USA 107, 14703–14708 (2010).
Hesseling, A. C. et al. Baseline sputum time to detection predicts month two culture conversion and relapse in non-HIV-infected patients. Int. J. Tuberc. Lung Dis. 14, 560–570 (2010).
Johnson, J. L. et al. Shortening treatment in adults with noncavitary tuberculosis and 2-month culture conversion. Am. J. Respir. Crit. Care Med. 180, 558–563 (2009).
Chee, C. B. et al. Tuberculosis treatment effect on T-cell interferon-γ responses to Mycobacterium tuberculosis-specific antigens. Eur. Respir. J. 36, 355–361 (2010).
Sai Priya, V. H., Latha, G. S., Hasnain, S. E., Murthy, K. J. & Valluri, V. L. Enhanced T cell responsiveness to Mycobacterium bovis BCG r32-kDa Ag correlates with successful anti-tuberculosis treatment in humans. Cytokine 52, 190–193 (2010).
Wassie, L. et al. Ex vivo cytokine mRNA levels correlate with changing clinical status of Ethiopian TB patients and their contacts over time. PLoS ONE 3, e1522 (2008).
Millington, K. A. et al. Dynamic relationship between IFN-γ and IL-2 profile of Mycobacterium tuberculosis-specific T cells and antigen load. J. Immunol. 178, 5217–5226 (2007).
Millington, K. A., Gooding, S., Hinks, T. S., Reynolds, D. J. & Lalvani, A. Mycobacterium tuberculosis-specific cellular immune profiles suggest bacillary persistence decades after spontaneous cure in untreated tuberculosis. J. Infect. Dis. 202, 1685–1689 (2010). This paper demonstrates that in some individuals T EM cells persist more than 50 years after spontaneous clinical cure of tuberculosis, suggesting the persistence of antigen. In some cured patients, however, only T CM cells are present, and this is consistent with sterilizing cure.
Bahk, Y. Y. et al. Antigens secreted from Mycobacterium tuberculosis: identification by proteomics approach and test for diagnostic marker. Proteomics 4, 3299–3307 (2004).
Berry, M. P. et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature 466, 973–977 (2010). This work illustrates a role for neutrophil-driven type I IFN signalling in M. tuberculosis pathogenesis and the importance of cell-specific transcriptome analysis.
Jacobsen, M. et al. Candidate biomarkers for discrimination between infection and disease caused by Mycobacterium tuberculosis. J. Mol. Med. 85, 613–621 (2007).
Mistry, R. et al. Gene-expression patterns in whole blood identify subjects at risk for recurrent tuberculosis. J. Infect. Dis. 195, 357–365 (2007).
Maertzdorf, J. et al. Human gene expression profiles of susceptibility and resistance in tuberculosis. Genes Immun. 12, 15–22 (2011).
Repsilber, D. et al. Biomarker discovery in heterogeneous tissue samples – taking the in-silico deconfounding approach. BMC Bioinformatics 11, 27 (2010).
Sartain, M. J., Slayden, R. A., Singh, K. K., Laal, S. & Belisle, J. T. Disease state differentiation and identification of tuberculosis biomarkers via native antigen array profiling. Mol. Cell. Proteomics 5, 2102–2113 (2006).
O'Connell, R. M., Rao, D. S., Chaudhuri, A. A. & Baltimore, D. Physiological and pathological roles for microRNAs in the immune system. Nature Rev. Immunol. 10, 111–122 (2010).
Liu, Q. et al. Serum protein profiling of smear-positive and smear-negative pulmonary tuberculosis using SELDI-TOF mass spectrometry. Lung 188, 15–23 (2010).
Agranoff, D. et al. Identification of diagnostic markers for tuberculosis by proteomic fingerprinting of serum. Lancet 368, 1012–1021 (2006).
de Carvalho, L. P. et al. Activity-based metabolomic profiling of enzymatic function: identification of Rv1248c as a mycobacterial 2-hydroxy-3-oxoadipate synthase. Chem. Biol. 17, 323–332 (2010).
Koulman, A., Lane, G. A., Harrison, S. J. & Volmer, D. A. From differentiating metabolites to biomarkers. Anal. Bioanal. Chem. 394, 663–670 (2009).
World Health Organisation. Treatment of tuberculosis: guidelines 4th edn. WHO [online], (2010).
Diel, R., Loddenkemper, R., Meywald-Walter, K., Niemann, S. & Nienhaus, A. Predictive value of a whole blood IFN-γ assay for the development of active tuberculosis disease after recent infection with Mycobacterium tuberculosis. Am. J. Respir. Crit. Care Med. 177, 1164–1170 (2008).
Doherty, T. M. et al. Immune responses to the Mycobacterium tuberculosis-specific antigen ESAT-6 signal subclinical infection among contacts of tuberculosis patients. J. Clin. Microbiol. 40, 704–706 (2002).
Mattos, A. M. et al. Increased IgG1, IFN-γ, TNF-α and IL-6 responses to Mycobacterium tuberculosis antigens in patients with tuberculosis are lower after chemotherapy. Int. Immunol. 22, 775–782 (2010).
Veenstra, H. et al. Changes in the kinetics of intracellular IFN-γ production in TB patients during treatment. Clin. Immunol. 124, 336–344 (2007).
Goletti, D. et al. Is IP-10 an accurate marker for detecting M. tuberculosis-specific response in HIV-infected persons? PLoS ONE 5, e12577 (2010).
Ruhwald, M. et al. Evaluating the potential of IP-10 and MCP-2 as biomarkers for the diagnosis of tuberculosis. Eur. Respir. J. 32, 1607–1615 (2008).
Ruhwald, M., Bjerregaard-Andersen, M., Rabna, P., Eugen-Olsen, J. & Ravn, P. IP-10, MCP-1, MCP-2, MCP-3, and IL-1RA hold promise as biomarkers for infection with M. tuberculosis in a whole blood based T-cell assay. BMC Res. Notes 2, 19 (2009).
Smith, S. G. et al. Mycobacterium tuberculosis PPD-induced immune biomarkers measurable in vitro following BCG vaccination of UK adolescents by multiplex bead array and intracellular cytokine staining. BMC Immunol. 11, 35 (2010).
Demissie, A. et al. Healthy individuals that control a latent infection with Mycobacterium tuberculosis express high levels of Th1 cytokines and the IL-4 antagonist IL-4δ2. J. Immunol. 172, 6938–6943 (2004).
Ordway, D. J. et al. Increased Interleukin-4 production by CD8 and γδ T cells in health-care workers is associated with the subsequent development of active tuberculosis. J. Infect. Dis. 190, 756–766 (2004).
Lee, J. H. & Chang, J. H. Changes of plasma interleukin-1 receptor antagonist, interleukin-8 and other serologic markers during chemotherapy in patients with active pulmonary tuberculosis. Korean J. Intern. Med. 18, 138–145 (2003).
Sutherland, J. S., de Jong, B. C., Jeffries, D. J., Adetifa, I. M. & Ota, M. O. Production of TNF-α, IL-12(p40) and IL-17 can discriminate between active TB disease and latent infection in a West African cohort. PLoS ONE 5, e12365 (2010).
Djoba Siawaya, J. F. et al. Differential expression of interleukin-4 (IL-4) and IL-4δ2 mRNA, but not transforming growth factor beta (TGF-β), TGF-βRII, Foxp3, gamma interferon, T-bet, or GATA-3 mRNA, in patients with fast and slow responses to antituberculosis treatment. Clin. Vaccine Immunol. 15, 1165–1170 (2008).
Djoba Siawaya, J. F. et al. Immune parameters as markers of tuberculosis extent of disease and early prediction of anti-tuberculosis chemotherapy response. J. Infect. 56, 340–347 (2008).
Eugen-Olsen, J. et al. The serum level of soluble urokinase receptor is elevated in tuberculosis patients and predicts mortality during treatment: a community study from Guinea-Bissau. Int. J. Tuberc. Lung Dis. 6, 686–692 (2002).
Demir, T., Yalcinoz, C., Keskinel, I., Demiroz, F. & Yildirim, N. sICAM-1 as a serum marker in the diagnosis and follow-up of treatment of pulmonary tuberculosis. Int. J. Tuberc. Lung Dis. 6, 155–159 (2002).
Mukae, H. et al. Elevated levels of circulating adhesion molecules in patients with active pulmonary tuberculosis. Respirology 8, 326–331 (2003).
Chan, C. H., Lai, C. K., Leung, J. C., Ho, A. S. & Lai, K. N. Elevated interleukin-2 receptor level in patients with active pulmonary tuberculosis and the changes following anti-tuberculosis chemotherapy. Eur. Respir. J. 8, 70–73 (1995).
Tsao, T. C. et al. Imbalances between tumor necrosis factor-α and its soluble receptor forms, and interleukin-1β and interleukin-1 receptor antagonist in BAL fluid of cavitary pulmonary tuberculosis. Chest 117, 103–109 (2000).
Djoba Siawaya, J. F., Ruhwald, M., Eugen-Olsen, J. & Walzl, G. Correlates for disease progression and prognosis during concurrent HIV/TB infection. Int. J. Infect. Dis. 11, 289–299 (2007).
Rosas-Taraco, A. G. et al. Expression of CDllc in blood monocytes as biomarker for favorable response to antituberculosis treatment. Arch. Med. Res. 40, 128–131 (2009).
Wolday, D. et al. Expression of chemokine receptors CCR5 and CXCR4 on CD4+ T cells and plasma chemokine levels during treatment of active tuberculosis in HIV-1-coinfected patients. J. Acquir. Immune Defic. Syndr. 39, 265–271 (2005).
Hosp, M. et al. Neopterin, β2-microglobulin, and acute phase proteins in HIV-1-seropositive and -seronegative Zambian patients with tuberculosis. Lung 175, 265–275 (1997).
Schleicher, G. K. et al. Procalcitonin and C-reactive protein levels in HIV-positive subjects with tuberculosis and pneumonia. Eur. Respir. J. 25, 688–692 (2005).
Krenke, R. & Korczynski, P. Use of pleural fluid levels of adenosine deaminase and interferon γ in the diagnosis of tuberculous pleuritis. Curr. Opin. Pulm. Med. 16, 367–375 (2010).
Abel, B. et al. The novel tuberculosis vaccine, AERAS-402, induces robust and polyfunctional CD4+ and CD8+ T cells in adults. Am. J. Respir. Crit. Care Med. 181, 1407–1417 (2010).
Hohn, H. et al. MHC class II tetramer guided detection of Mycobacterium tuberculosis-specific CD4+ T cells in peripheral blood from patients with pulmonary tuberculosis. Scand. J. Immunol. 65, 467–478 (2007).
Axelsson-Robertson, R. et al. Extensive major histocompatibility complex class I binding promiscuity for Mycobacterium tuberculosis TB10.4 peptides and immune dominance of human leucocyte antigen (HLA)-B*0702 and HLA-B*0801 alleles in TB10.4 CD8 T-cell responses. Immunology 129, 496–505 (2010).
Veenstra, H. et al. Changes in leucocyte and lymphocyte subsets during tuberculosis treatment; prominence of CD3dimCD56+ natural killer T cells in fast treatment responders. Clin. Exp. Immunol. 145, 252–260 (2006).
Azzurri, A. et al. Serological markers of pulmonary tuberculosis and of response to anti-tuberculosis treatment in a patient population in Guinea. Int. J. Immunopathol. Pharmacol. 19, 199–208 (2006).
Esquivel-Valerio, J. A. et al. Antineutrophil cytoplasm autoantibodies in patients with tuberculosis are directed against bactericidal/permeability increasing protein and are detected after treatment initiation. Clin. Exp. Rheumatol. 28, 35–39 (2010).
Jacobsen, M. et al. Suppressor of cytokine signaling (SOCS)-3 is affected in T cells from TB patients. Clin. Microbiol. Infect. 29 Jul 2010 (doi:10.1111/j.1469-0691.2010.03326.x).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Latent M. tuberculosis infection
-
Latent infection with M. tuberculosis indicates the presence of live M. tuberculosis organisms in a human host who is asymptomatic. It is detected by demonstrating immune responsiveness of the host to M. tuberculosis antigens (using the tuberculin skin test or interferon-γ release assays). Latent infection can last a lifetime.
- Active tuberculosis
-
The symptomatic disease caused by M. tuberculosis infection. Approximately 10% of infected individuals develop active disease in their lifetime owing to a loss of immune control over the pathogen. The disease manifests mainly in the lungs but can be extrapulmonary or disseminated.
- Tuberculosis biomarker
-
An ideal tuberculosis biomarker should: differentiate between patients with active tuberculosis and individuals with latent M. tuberculosis infection; return to normal levels during treatment; reproducibly predict clinical outcomes (for example, cure, relapse risk or eradication of M. tuberculosis infection) in diverse patient populations; and predict vaccine efficacy and provide end points for clinical trials.
- Sputum smear test
-
Quantification of mycobacteria in stained sputum preparations by microscopic examination. Traditionally, this test is used for diagnosis and after the 2-month intensive phase of tuberculosis treatment to assess treatment response.
- Sputum culture test
-
Assessment of the growth of M. tuberculosis from sputum in (currently mostly liquid) culture medium. Sputum culture conversion is used to assess treatment success. Successful treatment is determined by a lack of M. tuberculosis growth in a sample from an individual whose previous sputum culture test was positive.
- Correlates of risk
-
Markers whose presence is associated with a low risk of disease, or whose absence is associated with a high risk of disease.
- Correlates of protection
-
Several terms are used for this concept, including surrogates of protection. These markers reliably predict the level of protective efficacy induced by a vaccine on the basis of differences in the immunological measurements of vaccinated and unvaccinated groups.
- Effector memory T cell
-
A terminally differentiated T cell that lacks lymph node-homing receptors but expresses receptors that enable it to home to inflamed tissues. Effector memory T cells can exert immediate effector functions without the need for further differentiation.
- Central memory T cell
-
An antigen-experienced T cell that expresses cell-surface receptors for homing to secondary lymphoid organs. These cells are generally thought to be long-lived and can serve as the precursors for effector T cells in recall responses.
- Relapse
-
A recurrent episode of tuberculosis after initial cure, resulting from incomplete clearance of the original infection. The same bacterial strain is involved at both episodes.
- Tuberculin skin test reaction
-
A delayed-type hypersensitivity reaction following intradermal injection of purified M. tuberculosis-derived proteins. The tuberculin skin test is also known as the Mantoux test and is used as a diagnostic tool for latent M. tuberculosis infection.
- γδ T cells
-
T cells that express the γδ T cell receptor. These cells are present in the skin, vagina and intestinal epithelium as intraepithelial lymphocytes. Although the exact function of γδ T cells is unknown, it has been suggested that mucosal γδ T cells are involved in innate immune responses.
- Meta-analysis
-
A statistical approach that combines results from multiple related studies to define a composite effect. When applied to genome-wide association studies, more modest association effects can be identified.
- Baseline biomarkers
-
Markers that can be measured at diagnosis of tuberculosis disease before the commencement of treatment.
- Time-to-detection in liquid culture
-
The number of days until growth of M. tuberculosis is detected in liquid culture medium.
- Cured tuberculosis patient
-
A patient whose sputum smear tests (and sputum culture tests, if available) are negative in both the last month of treatment (conventionally month 6) and on at least one previous occasion. This does not necessarily equate to sterilizing cure.
- MicroRNAs
-
Small RNA molecules that regulate the expression of genes by binding to the 3′-untranslated regions (3′-UTRs) of specific mRNAs.
Rights and permissions
About this article
Cite this article
Walzl, G., Ronacher, K., Hanekom, W. et al. Immunological biomarkers of tuberculosis. Nat Rev Immunol 11, 343–354 (2011). https://doi.org/10.1038/nri2960
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri2960
This article is cited by
-
Blood monocyte and dendritic cell profiles among people living with HIV with Mycobacterium tuberculosis co-infection
BMC Immunology (2023)
-
Identification of host biomarkers from dried blood spots for monitoring treatment response in extrapulmonary tuberculosis
Scientific Reports (2023)
-
Plasma cytokine levels characterize disease pathogenesis and treatment response in tuberculosis patients
Infection (2023)
-
Production, characterization, and application of phage-derived PK34 recombinant anti-microbial peptide
Applied Microbiology and Biotechnology (2023)
-
Exploring the value of Mycobacterium tuberculosis modified lipoprotein as a potential biomarker for TB detection in children
BMC Infectious Diseases (2022)