Abstract
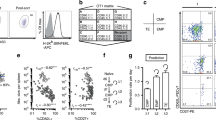
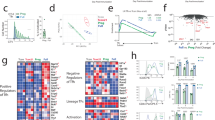
We studied here the long-term maintenance of distinct populations of T helper type 1 (TH1)-lineage cells in vivo and found that effector TH1 cells, defined by their secretion of interferon-γ (IFN-γ), are short-lived and do not efficiently develop into long-term memory TH1 cells. In contrast, a population of activated TH1-lineage cells that did not secrete IFN-γ after primary antigenic stimulation persisted for several months in vivo and developed the capacity to secrete IFN-γ upon subsequent stimulation. These data suggest that a linear differentiation pathway, as defined by the transition from IFN-γ–producing to resting memory cells, is relatively limited in vivo and support a revised model for TH1 memory differentiation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Abbas, A.K., Murphy, K.M. & Sher, A. Functional diversity of helper T lymphocytes. Nature 383, 787–793 (1996).
O'Garra, A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity 8, 275–283 (1998).
Murphy, K.M. et al. Signaling and transcription in T helper development. Annu. Rev. Immunol. 18, 451–494 (2000).
Mullen, A.C. et al. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science 292, 1907–1910 (2001).
Ahmed, R. & Gray, D. Immunological memory and protective immunity: understanding their relation. Science 272, 54–60 (1996).
Dutton, R.W., Bradley, L.M. & Swain, S.L. T cell memory. Annu. Rev. Immunol. 16, 201–223 (1998).
Sprent, J. & Surh, C.D. T cell memory. Annu. Rev. Immunol. 20, 551–579 (2002).
Swain, S.L. Generation and in vivo persistence of polarized TH1 and TH2 memory cells. Immunity 1, 543–552 (1994).
Swain, S.L., Hu, H. & Huston, G. Class II-independent generation of CD4 memory T cells from effectors. Science 286, 1381–1383 (1999).
Trinchieri, G. Immunobiology of interleukin-12. Immunol. Res. 17, 269–278 (1998).
Stobie, L. et al. The role of antigen and IL-12 in sustaining TH1 memory cells in vivo: IL-12 is required to maintain memory/effector TH1 cells sufficient to mediate protection to an infectious parasite challenge. Proc. Natl. Acad. Sci. USA 97, 8427–8432 (2000).
Park, A.Y., Hondowicz, B.D. & Scott, P. IL-12 is required to maintain a TH1 response during Leishmania major infection. J. Immunol. 165, 896–902 (2000).
Yap, G., Pesin, M. & Sher, A. IL-12 is required for the maintenance of IFN-γ production in T cells mediating chronic resistance to the intracellular pathogen, Toxoplasma gondii. J. Immunol. 165, 628–631 (2000).
Leonard, J.P., Waldburger, K.E. & Goldman, S.J. Prevention of experimental autoimmune encephalomyelitis by antibodies against interleukin 12. J. Exp. Med. 181, 381–386 (1995).
Neurath, M.F., Fuss, I., Kelsall, B.L., Stuber, E. & Strober, W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J. Exp. Med. 182, 1281–1290 (1995).
Tarrant, T.K., Silver, P.B., Chan, C.C., Wiggert, B. & Caspi, R.R. Endogenous IL-12 is required for induction and expression of experimental autoimmune uveitis. J. Immunol. 161, 122–127 (1998).
Hong, K., Berg, E.L. & Ehrhardt, R.O. Persistence of pathogenic CD4+ TH1-like cells in vivo in the absence of IL-12 but in the presence of autoantigen. J. Immunol. 166, 4765–4772 (2001).
Mendel, I. & Shevach, E.M. Differentiated TH1 autoreactive effector cells can induce experimental autoimmune encephalomyelitis in the absence of IL-12 and CD40/CD40L interactions. J. Neuroimmunol. 122, 65–73 (2002).
Hu-Li, J., Huang, H., Ryan, J. & Paul, W.E. In differentiated CD4+ T cells, interleukin 4 production is cytokine-autonomous, whereas interferon γ production is cytokine dependent. Proc. Natl. Acad. Sci. USA 94, 3189–3194 (1997).
Rogers, P.R., Dubey, C. & Swain, S.L. Qualitative changes accompany memory T cell generation: faster, more effective responses at lower doses of antigen. J. Immunol. 164, 2338–2346 (2000).
Openshaw, P. et al. Heterogeneity of intracellular cytokine synthesis at the single-cell level in polarized T helper 1 and T helper 2 populations. J. Exp. Med. 182, 1357–1367 (1995).
Harbertson, J., Biederman, E., Bennett, K.E., Kondrack, R.M. & Bradley, L.M. Withdrawal of stimulation may initiate the transition of effector to memory CD4 cells. J. Immunol. 168, 1095–1102 (2002).
Ahmadzadeh, M., Hussain, S.F. & Farber, D.L. Heterogeneity of the memory CD4 T cell response: persisting effectors and resting memory T cells. J. Immunol. 166, 926–935 (2001).
Bucy, R.P. et al. Single cell analysis of cytokine gene coexpression during CD4+ T-cell phenotype development. Proc. Natl. Acad. Sci. USA 92, 7565–7569 (1995).
Scheffold, A. et al. Analysis and sorting of T cells according to cytokine expression. Eur. Cytokine Netw. 9, 5–11 (1998).
Iezzi, G., Scheidegger, D. & Lanzavecchia, A. Migration and function of antigen-primed nonpolarized T lymphocytes in vivo. J. Exp. Med. 193, 987–993 (2001).
Hu, H. et al. CD4+ T cell effectors can become memory cells with high efficiency and without further division. Nature Immunol. 2, 705–710 (2001).
Reinhardt, R.L., Khoruts, A., Merica, R., Zell, T. & Jenkins, M.K. Visualizing the generation of memory CD4 T cells in the whole body. Nature 410, 101–105 (2001).
Masopust, D., Vezys, V., Marzo, A.L. & Lefrancois, L. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291, 2413–2417 (2001).
Sallusto, F., Lenig, D., Forster, R., Lipp, M. & Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401, 708–712 (1999).
Szabo, S.J. et al. A novel transcription factor, T-bet, directs TH1 lineage commitment. Cell 100, 655–669 (2000).
Szabo, S.J., Dighe, A.S., Gubler, U. & Murphy, K.M. Regulation of the interleukin (IL)-12Rβ2 subunit expression in developing T helper 1 (TH1) and TH2 cells. J. Exp. Med. 185, 817–824 (1997).
Zheng, W. & Flavell, R.A. The transcription factor GATA-3 is necessary and sufficient for TH2 cytokine gene expression in CD4 T cells. Cell 89, 587–596 (1997).
Dalton, D.K. et al. Multiple defects of immune cell function in mice with disrupted interferon-γ genes. Science 259, 1739–1742 (1993).
Zhang, X. et al. Unequal death in T helper cell (TH)1 and TH2 effectors: TH1, but not TH2, effectors undergo rapid Fas/FasL-mediated apoptosis. J. Exp. Med. 185, 1837–1849 (1997).
Opferman, J.T., Ober, B.T. & Ashton-Rickardt, P.G. Linear differentiation of cytotoxic effectors into memory T lymphocytes. Science 283, 1745–1748 (1999).
Jacob, J. & Baltimore, D. Modelling T-cell memory by genetic marking of memory T cells in vivo. Nature 399, 593–597 (1999).
Homann, D., Teyton, L. & Oldstone, M.B. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nature Med. 7, 913–919 (2001).
Gett, A.V. & Hodgkin, P.D. Cell division regulates the T cell cytokine repertoire, revealing a mechanism underlying immune class regulation. Proc. Natl. Acad. Sci. USA 95, 9488–9493 (1998).
Bird, J.J. et al. Helper T cell differentiation is controlled by the cell cycle. Immunity 9, 229–237 (1998).
Richter, A., Lohning, M. & Radbruch, A. Instruction for cytokine expression in T helper lymphocytes in relation to proliferation and cell cycle progression. J. Exp. Med. 190, 1439–1450 (1999).
Grogan, J.L. et al. Early transcription and silencing of cytokine genes underlie polarization of T helper cell subsets. Immunity 14, 205–215 (2001).
Wang, X. & Mosmann, T. In vivo priming of CD4 T cells that produce interleukin (IL)-2 but not IL-4 or interferon (IFN)-γ, and can subsequently differentiate into IL-4- or IFN-γ-secreting cells. J. Exp. Med. 194, 1069–1080 (2001).
Panus, J.F., McHeyzer-Williams, L.J. & McHeyzer-Williams, M.G. Antigen-specific T helper cell function: differential cytokine expression in primary and memory responses. J. Exp. Med. 192, 1301–1316 (2000).
Hayashi, N., Liu, D., Min, B., Ben-Sasson, S.Z. & Paul, W.E. Antigen challenge leads to in vivo activation and elimination of highly polarized TH1 memory T cells. Proc. Natl. Acad. Sci. USA 99, 6187–6191 (2002).
Gurunathan, S., Prussin, C., Sacks, D.L. & Seder, R.A. Vaccine requirements for sustained cellular immunity to an intracellular parasitic infection. Nature Med. 4, 1409–1415 (1998).
Hou, S., Hyland, L., Ryan, K.W., Portner, A. & Doherty, P.C. Virus-specific CD8+ T-cell memory determined by clonal burst size. Nature 369, 652–654 (1994).
Huygen, K. et al. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nature Med. 2, 893–898 (1996).
Rhee, E.G. et al. Vaccination with heat-killed leishmania antigen or recombinant leishmanial protein and CpG oligodeoxynucleotides induces long-term memory CD4+ and CD8+ T cell responses and protection against Leishmania major infection. J. Exp. Med. 195, 1565–1573 (2002).
Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, 45–45 (2001).
Acknowledgements
We thank B. Marshal for editorial assistance and X. Tai for technical help with cell preparation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Wu, Cy., Kirman, J., Rotte, M. et al. Distinct lineages of TH1 cells have differential capacities for memory cell generation in vivo. Nat Immunol 3, 852–858 (2002). https://doi.org/10.1038/ni832
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni832
This article is cited by
-
T-cell tolerance and exhaustion in the clearance of Echinococcus multilocularis: role of inoculum size in a quantitative hepatic experimental model
Scientific Reports (2017)
-
Alphavirus-based Vaccines Encoding Nonstructural Proteins of Hepatitis C Virus Induce Robust and Protective T-cell Responses
Molecular Therapy (2014)
-
A detailed phenotypic analysis of immune cell populations in the bronchoalveolar lavage fluid of atopic asthmatics after segmental allergen challenge
Allergy, Asthma & Clinical Immunology (2013)
-
Resolution of infection promotes a state of dormancy and long survival of CD4 memory T cells
Immunology & Cell Biology (2011)
-
Intranasal immunization with plasmid DNA encoding spike protein of SARS-coronavirus/polyethylenimine nanoparticles elicits antigen-specific humoral and cellular immune responses
BMC Immunology (2010)