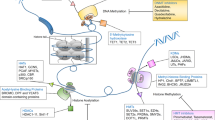
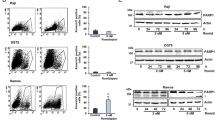
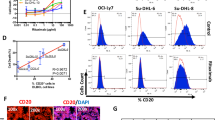
Abstract
Reversible acetylation is mediated by histone deacetylase (HDAC), which is involved in regulating a broad repertoire of physiological processes, many of which are under aberrant control in tumor cells. Inhibition of HDAC activity prompts tumor cells to enter apoptosis; therefore, the utility of HDAC inhibitors for the treatment of cancer has been investigated and several HDAC inhibitors have now entered clinical trials. Although the clinical picture is evolving and the precise clinical utility of HDAC inhibitors remains to be determined, it is noteworthy that certain tumor types have a favorable response to such agents. Hematological malignancies seem to be particularly sensitive, and vorinostat (also called suberoylanilide hydroxamic acid) has recently been approved for the treatment of cutaneous manifestations of cutaneous T-cell lymphoma in patients with progressive, persistent or recurrent disease. There are considerable gaps in our understanding of how HDAC inhibitors exert their antitumor activity. In the absence of mechanistic insights into the apoptotic process or biomarkers that inform on responsive tumors, it is a challenge to predict tumor response to HDAC-inhibitor-based therapies with any degree of certainty. In this Review, we discuss recent developments in the understanding of the molecular events that underlie the anticancer effects of HDAC inhibitors, and relate this information to the emerging clinical picture for the treatment of cutaneous T-cell lymphoma and related malignancies.
Key Points
-
Reversible acetylation of histones on chromatin is an important level of epigenetic control, and it is becoming increasingly clear that acetylation affects many of the key processes that drive tumorigenesis, including the cell cycle, apoptosis, angiogenesis and invasion
-
Histone deacetylases (HDACs) are a family of related enzymes that regulate the level of lysine acetylation on substrate proteins, which is frequently aberrant in tumor cells, whereas histone acetyl transferases add acetyl groups to lysine residues
-
Small-molecule inhibitors of HDAC cause tumor cells to enter apoptosis; however, it is not known with any degree of certainty how inhibition of HDAC activity causes tumor-cell death, although several mechanisms have been proposed
-
At the moment, only one HDAC inhibitor, vorinostat, has been approved by the FDA for the treatment cutaneous T-cell lymphoma, on the basis of a phase II study in patients with progressive disease following two systemic therapies
-
Although the clinical efficacy of HDAC inhibitors for cutaneous T-cell lymphoma has been demonstrated in phase II proof-of-concept clinical trials, this utility has not been identified for other tumors; consequently, there is a need to identify biomarkers that determine tumors likely to have a favorable clinical response
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ting AH et al. (2006) The cancer epigenome—components and functional correlates. Genes Dev 20: 3215–3231
Inche AG and La Thangue NB (2006) Chromatin control and cancer-drug discovery: realizing the promise. Drug Discov Today 11: 97–109
Carey N and La Thangue NB (2006) Histone deacetylase inhibitors: gathering pace. Curr Opin Pharmacol 6: 369–375
Marks P et al. (2001) Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer 1: 194–202
Marks PA et al. (2004) Histone deacetylase inhibitors. Adv Cancer Res 91: 137–168
Marks PA and Breslow R (2007) Dimethyl sulfoxide to vorinostat: development of this histone deacetylase inhibitor as an anticancer drug. Nat Biotechnol 25: 84–90
Duvic M and Zhang C (2006) Clinical and laboratory experience of vorinostat (suberoylanilide hydroxamic acid) in the treatment of cutaneous T-cell lymphoma. Br J Cancer 95: S13–S19
Luger K et al. (1997) Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature 389: 251–260
Jenuwein T and Allis CD (2001) Translating the histone code. Science 293: 1074–1080
Spotswood HT and Turner BM (2002) An increasingly complex code. J Clin Invest 110: 577–582
Chan HM et al. (2001) Acetylation control of the retinoblastoma tumour-suppressor protein. Nat Cell Biol 3: 667–674
Bolden JE et al. (2006) Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov 5: 769–784
de Ruijter AJ et al. (2003) Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J 370: 737–749
Yoshida M et al. (1990) Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J Biol Chem 265: 17174–17179
Finnin MS et al. (1999) Structures of a histone deacetylase homologue bound to the TSA and SAHA inhibitors. Nature 401: 188–193
Glaser KB et al. (2003) Gene expression profiling of multiple histone deacetylase (HDAC) inhibitors: defining a common gene set produced by HDAC inhibition in T24 and MDA carcinoma cell lines. Mol Cancer Ther 2: 151–163
Johnstone RW and Licht JD (2003) Histone deacetylase inhibitors in cancer therapy: is transcription the primary target? Cancer Cell 4: 13–18
Mitsiades CS et al. (2004) Transcriptional signature of histone deacetylase inhibition in multiple myeloma: biological and clinical implications. Proc Natl Acad Sci USA 101: 540–545
Dokmanovic M et al. (2007) Histone deacetylase inhibitors: overview and perspectives. Mol Cancer Res 5: 981–989
Kovacs JJ et al. (2005) HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Mol Cell 18: 601–607
Gu W and Roeder RG (1997) Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell 90: 595–606
Martinez-Balbas MA et al. (2000) Regulation of E2F1 activity by acetylation. EMBO J 19: 662–671
Patel JH et al. (2004) The c-MYC oncoprotein is a substrate of the acetyltransferases hGCN5/PCAF and TIP60. Mol Cell Biol 24: 10826–10834
Billin AN et al. (2000) Beta-catenin-histone deacetylase interactions regulate the transition of LEF1 from a transcriptional repressor to an activator. Mol Cell Biol 20: 6882–6890
Park JH et al. (2004) Class I histone deacetylase-selective novel synthetic inhibitors potently inhibit human tumor proliferation. Clin Cancer Res 10: 5271–5281
Huang BH et al. (2005) Inhibition of histone deacetylase 2 increases apoptosis and p21Cip1/WAF1 expression, independent of histone deacetylase 1. Cell Death Differ 12: 395–404
Zhang Y et al. (2003) HDAC-6 interacts with and deacetylates tubulin and microtubules in vivo. EMBO J 22: 1168–1179
Kawaguchi Y et al. (2003) The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress. Cell 115: 727–738
Boyault C et al. (2006) HDAC6-p97/VCP controlled polyubiquitin chain turnover. EMBO J 25: 3357–3366
Zhang CL et al. (2002) Class II histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell 110: 479–488
Vega K et al. (2004) Histone deacetylase 4 controls chondrocyte hypertrophy during skeletogenesis. Cell 119: 555–566
Arnold MA et al. (2007) MEF2C transcription factor controls chondrocyte hypertrophy and bone development. Dev Cell 12: 377–389
Dequiedt F et al. (2003) HDAC7, a thymus-specific class II histone deacetylase, regulates Nur77 transcription and TCR-mediated apoptosis. Immunity 18: 687–698
Zhu E et al. (2004) Induction of HDAC2 expression upon loss of APC in colorectal tumorigenesis. Cancer Cell 5: 455–463
Ropero S et al. (2006) A truncating mutation of HDAC2 in human cancers confers resistance to histone deacetylase inhibition. Nat Genet 38: 566–569
Minucci S and Pelicci PG (2006) Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer 6: 38–51
Wu WS et al. (2001) The growth suppressor PML represses transcription by functionally and physically interacting with histone deacetylases. Mol Cell Biol 21: 2259–2268
Reddy P et al. (2004) Histone deacetylase inhibitor suberoylanilide hydroxamic acid reduces acute graft-versus-host disease and preserves graft-versus-leukaemia effect. Proc Natl Acad Sci USA 101: 3921–3926
Brogdon JL et al. (2006) Histone deacetylase activities are required for innate immune cell control of Th1 but Th2 effector cell function. Blood 109: 1123–1130
Tao R et al. (2007) Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med 13: 1299–1307
Shao Y et al. (2004) Apoptotic and autophagic cell death induced by histone deacetylase inhibitors. Proc Natl Acad Sci USA 101: 18030–18035
Yang X et al. (2001) Synergistic activation of functional estrogen receptor (ER)-alpha by DNA methyltransferase and histone deacetylase inhibition in human ER-alpha-negative breast cancer cells. Cancer Res 61: 7025–7029
Ferrara FF et al. (2001) Histone deacetylase-targeted treatment restores retinoic acid signaling and differentiation in acute myeloid leukemia. Cancer Res 61: 2–7
Rahmani M et al. (2003) Coadministration of the heat shock protein 90 antagonist 17-allylamino- 17-demethoxygeldanamycin with suberoylanilide hydroxamic acid or sodium butyrate synergistically induces apoptosis in human leukemia cells. Cancer Res 63: 8420–8427
Hideshima T et al. (2005) Small-molecule inhibition of proteasome and aggresome function induces synergistic antitumor activity in multiple myeloma. Proc Natl Acad Sci USA 102: 8567–8572
Tumber A et al. (2007) The histone deacetylase inhibitor PXD101 synergises with 5-fluorouracil to inhibit colon cancer cell growth in vitro and in vivo. Cancer Chemother Pharmacol 60: 275–283
Marchion DC et al. (2004) Sequence-specific potentiation of topoisomerase II inhibitors by the histone deacetylase inhibitor suberoylanilide hydroxamic acid. J Cell Biochem 92: 223–237
Grant S et al. (2007) Vorinostat. Nat Rev Drug Discov 6: 21–22
Glaser KB (2007) HDAC inhibitors: clinical update and mechanism-based potential. Biochem Pharmacol 74: 659–671
Keehn CA et al. (2007) The diagnosis, staging, and treatment options for mycosis fungoides. Cancer Control 14: 102–111
Chuang TY et al. (1990) Incidence of cutaneous T cell lymphoma and other rare skin cancers in a defined population. J Am Acad Dermatol 23: 254–256
Willemze R et al. (2005) WHO-EORTC classification for cutaneous lymphomas. Blood 105: 3768–3785
Lessin SR et al. (1994) Retroviruses and cutaneous T-cell lymphoma. Dermatol Clin 12: 243–253
Kim EJ et al. (2006) Mycosis fungoides and Sézary syndrome: an update. Curr Oncol Rep 8: 376–386
van Doorn R et al. (2002) A novel splice variant of the Fas gene in patients with cutaneous T-cell lymphoma. Cancer Res 62: 5389–5392
Sommer VH et al. (2004) In vivo activation of STAT3 in cutaneous T-cell lymphoma: evidence for an antiapoptotic function of STAT3. Leukemia 18: 1288–1295
Lauritzen AF et al. (1995) p53 protein expression in cutaneous T-cell lymphomas. Br J Dermatol 133: 32–36
Navas IC et al. (2000) p16(INK4a) gene alterations are frequent in lesions of mycosis fungoides. Am J Pathol 156: 1565–1572
Scarisbrick JJ et al. (2002) Frequent abnormalities of the p15 and p16 genes in mycosis fungoides and Sézary syndrome. J Invest Dermatol 118: 493–499
Scarisbrick JJ et al. (2003) Microsatellite instability is associated with hypermethylation of the hMLH1 gene and reduced gene expression in mycosis fungoides. J Invest Dermatol 121: 894–901
Zhang C et al. (2007) Consequences of p16 tumor suppressor gene inactivation in mycosis fungoides and Sézary syndrome and role of the bmi-1 and ras oncogenes in disease progression. Hum Pathol 38: 995–1002
Sherr CJ and Roberts JM (1999) CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev 13: 1501–1512
van Doorn R et al. (2005) Epigenetic profiling of cutaneous T-cell lymphoma: promoter hypermethylation of multiple tumor suppressor genes including BCL7a, PTPRG, and p73. J Clin Oncol 23: 3886–3896
Alizadeh AA et al. (2000) Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 403: 503–511
Martinez-Delgado B et al. (2002) Frequent inactivation of the p73 gene by abnormal methylation or LOH in non-Hodgkin's lymphomas. Int J Cancer 102: 15–19
Siu LL et al. (2003) Aberrant promoter CpG methylation as a molecular marker for disease monitoring in natural killer cell lymphomas. Br J Haematol 122: 70–77
Querfeld C et al. (2003) Primary cutaneous lymphomas: a review with current treatment options. Blood Rev 17: 131–142
Bunn PA Jr and Lamberg SI (1979) Report of the Committee on Staging and Classification of Cutaneous T-Cell Lymphomas. Cancer Treat Rep 63: 725–728
Sausville EA et al. (1988) Histopathologic staging at initial diagnosis of mycosis fungoides and the Sézary syndrome: definition of three distinctive prognostic groups. Ann Intern Med 109: 372–382
Trautinger F et al. (2006) EORTC consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome. Eur J Cancer 42: 1014–1030
Budgin JB et al. (2005) Biological effects of bexarotene in cutaneous T-cell lymphoma. Arch Dermatol 141: 315–321
Zhang C et al. (2002) Induction of apoptosis by bexarotene in cutaneous T-cell lymphoma cells: relevance to mechanism of therapeutic action. Clin Cancer Res 8: 1234–1240
Duvic M et al. (2001) Bexarotene is effective and safe for treatment of refractory advanced-stage cutaneous T-cell lymphoma: multinational phase II-III trial results. J Clin Oncol 19: 2456–2471
vanderSpek JC et al. (1993) Structure/function analysis of the transmembrane domain of DAB389-interleukin-2, an interleukin-2 receptor-targeted fusion toxin: the amphipathic helical region of the transmembrane domain is essential for the efficient delivery of the catalytic domain to the cytosol of target cells. J Biol Chem 268: 12077–12082
Foss FM et al. (2001) Biological correlates of acute hypersensitivity events with DAB(389)IL-2 (denileukin diftitox, ONTAK) in cutaneous T-cell lymphoma: decreased frequency and severity with steroid premedication. Clin Lymphoma 1: 298–302
Olsen E et al. (2001) Pivotal phase III trial of two dose levels of denileukin diftitox for the treatment of cutaneous T-cell lymphoma. J Clin Oncol 19: 376–388
Kim EJ et al. (2005) Immunopathogenesis and therapy of cutaneous T cell lymphoma. J Clin Invest 115: 798–812
Mann BS et al. (2007) Vorinostat for treatment of cutaneous manifestations of advanced primary cutaneous T-cell lymphoma. Clin Cancer Res 13: 2318–2322
Piekarz RL et al. (2001) Inhibitor of histone deacetylation, depsipeptide (FR901228), in the treatment of peripheral and cutaneous T-cell lymphoma: a case report. Blood 98: 2865–2868
Kelly WK et al. (2005) Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J Clin Oncol 23: 3923–3931
O'Connor OA et al. (2006) Clinical experience with intravenous and oral formulations of the novel histone deacetylase inhibitor suberoylanilide hydroxamic acid in patients with advanced hematologic malignancies. J Clin Oncol 24: 166–173
Duvic M et al. (2007) Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL). Blood 109: 31–39
Olsen EA et al. (2007) Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J Clin Oncol 25: 3109–3115
Traynor AM et al. (2007) A phase II study of vorinostat (NSC 701852) in patients (pts) with relapsed non-small cell lung cancer (NSCLC). J Clin Oncol 25 (Suppl): 18044
Galanis E et al. (2007) N047B: NCCTG phase II trial of vorinostat (suberoylanilide hydroxamic acid) in recurrent glioblastoma multiforme (GBM). J Clin Oncol 25 (Suppl): 2004
Hussain M et al. (2007) Suberoylanilide hydroxamic acid (vorinostat) post chemotherapy in hormone refractory prostate cancer (HRPC) patients (pts): A phase II trial by the Prostate Cancer Clinical Trials Consortium (NCI 6862). J Clin Oncol 25 (Suppl): 5132
Krug LM et al. (2006) Potential role of histone deacetylase inhibitors in mesothelioma: clinical experience with suberoylanilide hydroxamic acid. Clin Lung Cancer 7: 257–261
Piekarz R et al. (2007) Update of the NCI multiinstitutional phase II trial of romidepsin, FK228, for patients with cutaneous or peripheral T-cell lymphoma. J Clin Oncol 25 (Suppl): 8027
Lerner A et al. (2006) Romidepsin (depsipeptide, FK228) induces clinically significant responses in treatment-refractory CTCL: interim report of a phase II multicenter study [abstract #2468]. Blood 108: 699a
Parker C et al. (2007) Romidepsin (FK228), a histone deacetylase inhibitor: Final results of a phase II study in metastatic hormone refractory prostate cancer (HRPC). J Clin Oncol 25 (Suppl): 15507
Molife R et al. (2006) Phase II study of FK228 in patients with metastatic hormone refractory prostate cancer (HRPC). J Clin Oncol 24 (Suppl): 14554
Sullivan D et al. (2006) A phase II study of PXD101 in advanced multiple myeloma [poster 3583]. Presented at the American Society of Hematology Annual Meeting: 2006 December 10–13, Orlando, FL
Prince M et al. (2006) LBH589, a novel deacetylase inhibitor (DACi), treatment of patients with cutaneous T cell lymphoma (CTCL): skin expression profiles in the first 24 h related to clinical response following therapy [abstract #2715]. Blood 108 (ASH Meeting Abstracts)
Younes A et al. (2007) A phase II study of a novel oral isotype-selective histone deacetylase (HDAC) inhibitor in patients with relapsed or refractory Hodgkin lymphoma. J Clin Oncol 25 (Suppl): 8000
Methylgene (online 21 August 2007) Methylgene and Pharmion announce US orphan drug designation granted for mgcd0103 for the treatment of Hodgkin's lymphoma [http://www.methylgene.com/content.asp?node=267] (accessed 13 February 2008)
Zhang C et al. (2005) Selective induction of apoptosis by histone deacetylase inhibitor SAHA in cutaneous T-cell lymphoma cells: relevance to mechanism of therapeutic action. J Invest Dermatol 125: 1045–1052
Lee JH et al. (2006) Histone deacetylase inhibitor enhances 5-fluorouracil cytotoxicity by down-regulating thymidylate synthase in human cancer cells. Mol Cancer Ther 5: 3085–3095
Zhou DC et al. (2002) Frequent mutations in the ligand-binding domain of PML-RAR alpha after multiple relapses of acute promyelocytic leukemia: analysis for functional relationship to response to all-trans retinoic acid and histone deacetylase inhibitors in vitro and in vivo. Blood 99: 1356–1363
Chung EJ et al. (2006) Assays for pharmacodynamic analysis of histone deacetylase inhibitors. Expert Opin Drug Metab Toxicol 2: 213–230
Acknowledgements
We thank Rosemary Williams for help in preparing the manuscript. Research in our laboratory is supported by Cancer Research UK, the Medical Research Council, the Association for International Cancer Research, the Leukaemia Research Fund and the European Union.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Khan, O., La Thangue, N. Drug Insight: histone deacetylase inhibitor-based therapies for cutaneous T-cell lymphomas. Nat Rev Clin Oncol 5, 714–726 (2008). https://doi.org/10.1038/ncponc1238
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncponc1238
This article is cited by
-
Inhibition of phospholipase D2 augments histone deacetylase inhibitor-induced cell death in breast cancer cells
Biological Research (2020)
-
A regulatory circuit that involves HR23B and HDAC6 governs the biological response to HDAC inhibitors
Cell Death & Differentiation (2013)
-
HDAC inhibitors in cancer biology: emerging mechanisms and clinical applications
Immunology & Cell Biology (2012)
-
Apoptosis Induction by SAHA in Cutaneous T-Cell Lymphoma Cells Is Related to Downregulation of c-FLIP and Enhanced TRAIL Signaling
Journal of Investigative Dermatology (2012)
-
Epigenetics in breast cancer: what's new?
Breast Cancer Research (2011)