Abstract
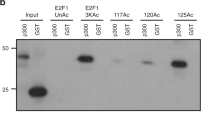
The retinoblastoma protein (Rb) silences specific genes that are active in the S phase of the cell cycle and which are regulated by E2F transcription factors1. Rb binds to the activation domain of E2F and then actively represses the promoter by a mechanism that is poorly understood2,3. Here we show that Rb associates with a histone deacetylase, HDAC1, through the Rb ‘pocket’ domain. Association with the deacetylase is reduced by naturally occurring mutations in the pocket and by binding of the human papilloma virus oncoprotein E7. We find that Rb can recruit histone deacetylase to E2F and that Rb cooperates with HDAC1 to repress the E2F-regulated promoter of the gene encoding the cell-cycle protein cyclin E. Inhibition of histone deacetylase activity by trichostatin A (TSA) inhibits Rb-mediated repression of a chromosomally integrated E2F-regulated promoter. Our results indicate that histone deacetylases are important for regulating the cell cycle and that active transcriptional repression by Rb may involve the modification of chromatin structure.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Weinberg, R. A. The retinoblastoma protein and cell cycle control. Cell 81, 323–330 (1995).
Weintraub, S. J., Prater, C. A. & Dean, D. C. Retinoblastoma protein switches the E2F site from positive to negative elements. Nature 358, 259–261 (1992).
Weintraub, S. J. et al. Mechanism of active transcriptional repression by the retinoblastoma protein. Nature 375, 812–815 (1995).
Taunton, J., Hassig, C. A. & Schreiber, S. L. Amammalian histone deacetylase related to the yeast transcriptional regulator Rpd3p. Science 272, 408–411 (1996).
Yang, W.-M., Inouye, C., Zeng, Y., Bearss, D. & Seto, E. Transcriptional repression by YY1 is mediated by interaction with a mammalian homolog of the yeast global regulator RPD2. Proc. Natl Acad. Sci. USA 93, 12845–12850 (1996).
Heinzel, T. et al. Acomplex containing N-CoR, mSin3 and histone deacetylase mediates transcriptional repression. Nature 387, 43–48 (1997).
Alland, L. et al. Role for N-CoR and histone deacetylase in Sin3-mediated transcriptional repression. Nature 387, 49–55 (1997).
Hassig, C. A., Fleischer, T. C., Billin, A. N., Schreiber, S. L. & Ayer, D. E. Histone deacetylase activity is required for full transcriptional repression by mSin3A. Cell 89, 341–347 (1997).
Laherty, C. D. et al. Histone deacetylases associated with the mSin3 corepressor mediate Mad transcriptional repression. Cell 89, 349–356 (1997).
Zhang, Y., Iratni, R., Erdjument-Bromage, H., Tempst, P. & Reinberg, D. Histone deacetylases and SAP18, a novel polypeptide, are components of a human Sin3 complex. Cell 89, 357–364 (1997).
Nagy, L. et al. Nuclear receptor repression mediated by a complex containing SMRT, mSin3A, and histone deacetylase. Cell 89, 373–380 (1997).
Kadosh, D. & Struhl, K. Repression by Ume6 involves recruitment of a complex containing Sin3 corepressor and Rpd3 histone deacetylase to target promoters. Cell 89, 365–371 (1997).
Qian, Y.-W. et al. Aretinoblastoma-binding protein related to a negative regulator of Ras in yeast. Nature 364, 648–652 (1993).
Huang, S., Lee, W.-H. & Lee, E. Y.-H. P. Acellular protein that competes with SV40 T antigen for binding to the retinoblastoma gene product. Nature 350, 160–162 (1991).
Hagemeier, C., Cook, A. & Kouzarides, T. The retinoblastoma protein binds E2F residues required for activation in vivo and TBP binding in vitro. Nucleic Acids Res. 21, 4998–5004 (1993).
Martin, K. et al. Stimualtion of E2F1/DP1 transcriptional activity by Mdm2 oncoprotein. Nature 375, 691–694 (1995).
Botz, J. et al. Cell cycle regulation of the murine cyclin E gene depends on an E2F binding site in the promoter. Mol. Cell Biol. 16, 3401–3409 (1996).
Hurford, R. K. J, Cobrinik, D., Lee, M. H. & Dyson, N. pRB and p107/p130 are required for the regulated expression of different sets of E2F responsive genes. Genes Dev. 11, 1447–1463 (1997).
Alevizopoulos, A. et al. Aproline-rich TGF-beta-responsive transcriptional activator interacts with histone H3. Genes Dev. 9, 3051–3066 (1995).
Bannister, A. J. & Kouzarides, T. The CBP co-activator is a histone acetyltransferase. Nature 384, 641–643 (1996).
Ogryzko, V. V., Schiltz, R. L., Russanova, V., Howard, B. H. & Nakatani, Y. The transcriptional coactivators p300 CBP are histone acetyltransferases. Cell 87, 953–959 (1996).
Gu, W. & Roeder, R. G. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell 90, 595–606 (1997).
Cavanaugh, A. H. et al. Activity of RNA polymerase I transcription factor UBF blocked by Rb gene product. Nature 374, 177–180 (1995).
White, R. J., Trouche, D., Martin, K., Jackson, S. P. & Kouzarides, T. Repression of RNA polymerase III transcription by the retinoblastoma protein. Nature 382, 88–90 (1996).
Larminie, C. G. et al. Mechanistic analysis of RNA polymerase III regulation by the retinoblastoma protein. EMBO J. 16, 2061–2071 (1997).
Acknowledgements
This work is dedicated to the memory of Danny Spiegelhalter who died of retinoblastoma. We thank C. Hassig and S. L. Schreiber for the 1121 and 1123 HCAC1 antibodies and for technical advice on peptide acetylation and histone deacetylase assay; S. Critchlow for the gift of XRCC4 protein; W. Zwerschke and P. Janssen-Duerr for the CycE reporter construct; N. Mermod for the 3T3/208 cell line and M. O'Connor for technical advice on GST microcolumns. This work was funded by a Cancer Research Campaign programme grant, by an MRC grant and by an EC Biomed2 grant. A.B. was supported by a grant from the Association for International Cancer Research; D.J.McC. was funded by an American Cancer Society International Cancer research fellowship. E.A.M. was supported by a CRC studentship; J.L.R. was funded by an MRC studentship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brehm, A., Miska, E., McCance, D. et al. Retinoblastoma protein recruits histone deacetylase to repress transcription. Nature 391, 597–601 (1998). https://doi.org/10.1038/35404
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35404
This article is cited by
-
Dysregulation of histone deacetylases in ocular diseases
Archives of Pharmacal Research (2024)
-
Acetyl-methyllysine marks chromatin at active transcription start sites
Nature (2023)
-
Establishment of H3K9-methylated heterochromatin and its functions in tissue differentiation and maintenance
Nature Reviews Molecular Cell Biology (2022)
-
Histone deacetylation primes self-propagation of heterochromatin domains to promote epigenetic inheritance
Nature Structural & Molecular Biology (2022)
-
The role of the DFF40/CAD endonuclease in genomic stability
Apoptosis (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.