Abstract
Introduction
Ventilator-associated pneumonia (VAP) is a common cause of nosocomial infection, and is related to significant utilization of health-care resources. In the past decade, new data have emerged about VAP epidemiology, diagnosis, treatment and prevention.
Results
Classifying VAP strictly based on time since hospitalization (early- and late-onset VAP) can potentially result in undertreatment of drug-resistant organisms in ICUs with a high rate of drug resistance, and overtreatment for patients not infected with resistant pathogens. A combined strategy incorporating diagnostic scoring systems, such as the Clinical Pulmonary Infection Score (CPIS), and either a quantitative or qualitative microbiological specimen, plus serial measurement of biomarkers, leads to responsible antimicrobial stewardship. The newly proposed ventilator-associated events (VAE) surveillance definition, endorsed by the Centers for Disease Control and Prevention, has low sensitivity and specificity for diagnosing VAP and the ability to prevent VAE is uncertain, making it a questionable surrogate for the quality of ICU care. The use of adjunctive aerosolized antibiotic treatment can provide high pulmonary concentrations of the drug and may facilitate shorter durations of therapy for multi-drug-resistant pathogens. A group of preventive strategies grouped as a ‘ventilator bundle’ can decrease VAP rates, but not to zero, and several recent studies show that there are potential barriers to implementation of these prevention strategies.
Conclusion
The morbidity and mortality related to VAP remain high and, in the absence of a gold standard test for diagnosis, suspected VAP patients should be started on antibiotics based on recommendations per the 2005 ATS guidelines and knowledge of local antibiotic susceptibility patterns. Using a combination of clinical severity scores, biomarkers, and cultures might help with reducing the duration of therapy and achieving antibiotic de-escalation.
Similar content being viewed by others
Introduction
Ventilator-associated pneumonia (VAP) is an important nosocomial infection that can complicate mechanical ventilation. The 2005 American Thoracic Society and Infectious Diseases Society of America (ATS/IDSA) guidelines provide a comprehensive approach to diagnosis and management of adults with nosocomial pneumonia [1], but in the past decade, new data have emerged about VAP epidemiology, diagnosis, treatment, and prevention.
The diagnosis of VAP is challenging and subject to considerable interobserver variability. The Centers for Disease Control and Prevention (CDC) definition of VAP uses a combination of clinical, radiographic, and microbiological criteria for diagnosis, but in the absence of a benchmark diagnostic test, the accurate diagnosis and treatment of VAP is limited. There has been a steady decline in reported VAP rates over the last several years in the USA, with the National Healthcare Safety Network (NHSN) reporting an incidence of VAP from 0.0 to 5.8 per 1,000 ventilator days [2]. It is unclear if this reduction in VAP incidence is the result of improved prevention measures or due to underestimation in a setting of public reporting to the Centers for Medicare and Medicaid Services (CMS) [3]. The CDC recently introduced a new concept—ventilator-associated events (VAE), a tiered approach as an objective surveillance measure to improve the accuracy of diagnosing ventilator-related complications [4]. However, the utility of VAE to identify all patients with pneumonia, the ability to prevent VAEs, and the role of VAEs in day to day clinical practice are uncertain.
The ATS/IDSA 2005 guidelines emphasize treatment based on the potential for developing infection with multidrug-resistant (MDR) pathogens. The microbiological agents and epidemiologic factors related to VAP have changed over the last decade and might influence treatment decisions and outcomes. The guidelines discussed invasive quantitative cultures as having some advantage over endotracheal aspirates for the diagnosis of VAP, but subsequent studies did not show any difference in mortality for either approach [5]. Recently, investigators have raised concerns about adherence to the guideline recommendation of using routine combination therapy for VAP [6, 7]. Prevention strategies based a group of interventions lumped into a ‘daily bundle’ have been shown to decrease the rate of VAP [8]. However, implementation has met with barriers in clinical practice, and the compliance rate with bundles is still modest.
There are several areas of uncertainty regarding VAP that require further clarification (as noted in Table 1). It is beyond the scope of this review to discuss all the topics, but we discuss the current understanding and controversies surrounding the major issues in VAP diagnosis, treatment, and prevention.
Changing epidemiology
VAP is generally divided into early onset or late onset (early, less than 5 days; late, more than 5 days after hospitalization), although some investigators have classified early-onset VAP as less than 7 days and late as more than 7 days since hospitalization [9, 10]. In previous guidelines, this classification has been related to bacteriology and empiric therapy choices, but recently, the bacteriologic differences between early- and late-onset VAP have been less clear, with some early-onset patients infected with drug-resistant pathogens, while certain patients in both groups can be infected with sensitive pathogens. Thus, antibiotic choice based on the time of pneumonia onset can lead to both over- and undertreatment with broad-spectrum agents.
Early- and late-onset VAP: is it important to make a distinction?
Traditionally, early-onset VAP was caused by drug-sensitive pathogens, such as Streptococcus pneumoniae, Haemophilus influenzae, and methicillin-sensitive Staphylococcus aureus, while late-onset VAP was caused by antibiotic-resistant pathogens, such as Pseudomonas aeruginosa, Acinetobacter spp., methicillin-resistant S. aureus (MRSA), and extended-spectrum beta-lactamase-producing Gram-negative bacilli. Recent studies have challenged these concepts (see Table 2).
In a large German database, the identity of the pathogens causing early- and late-onset VAP were similar, and the most frequent organisms were S. aureus, followed by P. aeruginosa, K. pneumoniae, and E. coli [11]. In another study, Restrepo and colleagues examined the cultures of 248 early-onset (less than 5 days) and 248 late-onset VAP (more than 5 days) patients and although patients with late-onset VAP had more significant antibiotic exposure in the prior month and higher Acute Physiology and Chronic Health Evaluation (APACHE) II scores on admission to ICU, both early- and late-onset VAP patients had similar rates of MDR pathogens (27.8 and 32.3 % respectively, p = 0.33) [12].
Ferrer and colleagues classified 276 patients with ICU-acquired pneumonia on the basis of time of onset and found that very few fell into group 1 (early onset without risk factors for MDR pathogens, n = 38, VAP in 18 patients), and that most were classified as group 2 (late onset or with risk factors for MDR pathogens, n = 238, VAP in 128 patients) [13]. Of the patients in group 1, 26 % had potentially drug-resistant pathogens isolated despite having no risk factors as per the 2005 guidelines. Investigators from the EU-VAP study group divided 485 patients with microbiology-confirmed nosocomial pneumonia into group 1, i.e., early onset with no MDR risk factors (n = 152), and group 2, i.e., early onset with MDR risk factors or late-onset pneumonia [14]. Of the patients in group 1, 50.7 % harbored resistant microorganisms including P. aeruginosa, S. maltophilia, MRSA, and Acinetobacter baumannii. Logistic regression analysis noted the presence of severe sepsis/septic shock (OR = 3.7) and pneumonia that developed in a center with greater than a 25 % prevalence of resistant pathogens (OR = 11.3) as independently associated with the presence of resistant pathogens in group 1 patients.
Thus classifying VAP patients based on time of onset might potentially result in undertreatment of those with early-onset infection and delayed institution of antibiotics and inadequate microbial coverage, both of which are related to adverse outcomes in patients with VAP [15–17]. On the other hand, empiric therapy can also result in overtreatment and thus therapy should be based on individual risks and the local prevalence of resistant pathogens.
Is there a difference between the etiologic agents causing VAP compared to those causing HAP?
The SENTRY antimicrobial surveillance program collected microbiological data from 1997 to 2008 in hospitalized patients with pneumonia from North America, Europe, and Latin America [18]. The top six pathogens isolated from patients with nosocomial pneumonia were S. aureus (28.0 %), P. aeruginosa (21.8 %), Klebsiella species (9.8 %), E. coli (6.9 %), Acinetobacter species (6.8 %), and Enterobacter species (6.3 %). There was no significant difference among the six common etiologic agents between patients with hospital-acquired pneumonia (HAP) and VAP. However, P. aeruginosa (26.6 %) and Acinetobacter species were more common in VAP than in HAP patients, and the incidence of S. aureus was lower among patients with VAP than among patients with HAP (19.5 vs. 26.6 %). Esperatti and colleagues prospectively compared 315 episodes of ICU-acquired pneumonia over a 3-year period in ventilated (n = 164) and non-ventilated patients (n = 151) [19]. There was no significant difference in the relative proportion of etiologic pathogens isolated in both the groups, except for S. pneumoniae, which was more common in non-ventilated ICU patients than VAP patients. In another prospective surveillance study comparing 327 episodes of VAP in 309 patients with 261 episodes of HAP in 247 patients, the patients with VAP had more episodes of Gram-negative bacilli, compared to patients with HAP (59 vs. 39.6 %, p < 0.001) and HAP patients had a higher incidence of S. pneumoniae and viruses [20].
Issues in the diagnosis of VAP
The CDC definition of VAP requires that patients be ventilated for more than 48 h and satisfy at least one radiographic, one systemic, and two pulmonary criteria [21] (see Table 3). Since many patients who are ventilated may develop other diseases that can mimic pneumonia, some investigators have recommended obtaining quantitative lower respiratory tract samples by bronchoscopic protected brush, bronchoalveolar lavage (BAL), or endotracheal aspiration in conjunction with the initiation of antibiotic treatment when VAP is suspected [22]. The clinical pulmonary infection score (CPIS) was developed to objectively diagnose VAP and assigns points on the basis of clinical and radiographic data, but its role in diagnosing pneumonia remains controversial [23]. This score was subsequently modified by Singh and colleagues to include radiographic progression and a score of 6 at baseline and at 72 h is considered suggestive of pneumonia (see Table 4) [24]. The ATS/IDSA 2005 guidelines have incorporated both a clinical and bacteriologic strategy for VAP diagnosis in their final recommendation.
Quantitative sampling of the lower respiratory tract and the role of clinical diagnosis of VAP based on CPIS
Although quantitative cultures improve the specificity of VAP diagnosis, there is a chance of false negative cultures in patients with partially treated and early pneumonia and false positive results with chronic colonization during prolonged mechanical ventilation. Ventilator-associated tracheobronchitis (VAT) is a related condition, characterized by clinical signs (fever, leukocytosis, and purulent sputum), microbiologic findings (Gram stain with bacteria and leukocytes, with either a positive semiquantitative or a quantitative sputum culture), but the absence of a new infiltrate on chest radiograph [25]. Patients with VAT have been shown to have prolonged duration of mechanical ventilation, ICU stay, and increased mortality [26]. However, it has been controversial whether recognition and early therapy of VAT can prevent the development of VAP. Some investigators have found that VAT is independent from VAP, while others have found that VAT is a predecessor of VAP [27, 28].
Heyland and colleagues randomly assigned 740 ventilated patients with suspected VAP to undergo either BAL with quantitative culture or endotracheal aspiration with non-quantitative culture of the aspirate and found no significant difference in mortality, length of stay in the hospital or ICU, and the frequency of targeted therapy/de-escalation with both approaches [5]. In a meta-analysis of four randomized control trials, there was no significant difference in mortality between an invasive bronchoscopic approach versus a non-invasive approach, but the invasive approach helped with antibiotic utilization and focusing therapy [29]. A recent Cochrane review revealed no significant differences in terms of mortality, length of ICU stay, duration of mechanical ventilation, and rate of antibiotic change between qualitative culture of noninvasive samples and quantitative culture of invasive samples [30].
The CPIS score was originally shown to have a good correlation with the bacterial index of samples obtained via BAL [23], but it has a low sensitivity in patients with ARDS and in surgical patients; however, if measured serially, the CPIS score may help guide the duration of antibiotic treatment [31]. The CPIS score can be more valuable if used in conjunction with a good lower respiratory tract sample, which includes Gram staining [32]. In another study, 299 mechanically ventilated patients had twice weekly surveillance endotracheal aspirate cultures (EA) [33], and in the 41 patients diagnosed with VAP by BAL, the CPIS score was significantly elevated compared to 34 patients with negative BAL culture (6.6 vs. 5, p = 0.001). The initial empiric antibiotic therapies based on the EA cultures were adequate in 95 % of patients with VAP. Of the various measures included in CPIS, oxygenation measured by the ratio of partial pressure of oxygen compared to fractional inspired oxygen (PaO2/FiO2) has consistently proven to be a good indicator of outcome, particularly when followed serially [34].
Several biomarkers, including soluble triggering receptor expressed on myeloid cells-1 (sTREM-1), procalcitonin (PCT), copeptin, and C-reactive protein (CRP), have been studied in patients with VAP. In a prospective study, serial PCT measurements on days 1, 3, and 7 were significantly higher in microbiologically proven VAP patients who had an unfavorable outcome than those with a favorable outcome, while serial CPIS values were less discriminatory [35]. However, in that study, serial measurements of oxygenation did distinguish patients with an unfavorable outcome from those with a good outcome. Ramirez and colleagues found that a combination of CPIS of at least 6 points and serum PCT levels of at least 2.99 ng/mL had 100 % specificity and negative predictive value of 92 % (AUC = 0.961) for the diagnosis of VAP in a small study (n = 44) [36]. Although clinical features are not specific, serial measurements of oxygenation and biomarkers might be the most useful strategy for deciding when to use antibiotics and when to de-escalate once a particular pathogen is identified.
What is the role of new surveillance definitions for VAP and VAE?
The CDC definition of VAP is subject to interobserver variability and subjectivity, and thus Klompas and colleagues developed a simplified streamlined surveillance definition of VAP and compared it to the conventional CDC definition in 600 patients [37]. They excluded the criteria of delirium and rales, and defined worsening oxygenation as at least 2 days of stable or decreasing daily minimum PEEP, followed by an increase of at least 2.5 cmH2O of PEEP for more than 2 days; or at least 2 days of stable or decreasing FiO2, followed by an increase of at least 0.15 in FiO2 sustained over 2 days. Compared to the conventional definition, the streamlined definition identified patients with VAP faster (3.5 vs. 39 min per patient) and more objectively, with no major difference in hospital mortality, ICU length of stay, and ventilator days between the two definitions. However, no chest radiograph was used in the streamlined definition, a feature that may be either a problem or an advantage.
To improve objectivity and reproducibility, a CDC working group recently proposed an alternative definition for VAE, which include ventilator-associated complications (VAC), infectious ventilator-associated complications (IVAC), and probable versus possible VAP (see Table 5) [38, 39]. The definitions focus on worsening oxygenation (defined by ventilator settings and not by physiologic measurements) and systemic signs of infection and exclude the use of chest radiography. Problems with this definition include the need for an initial 2 days of stability, thus excluding some early VAP, and dependence on changes in the FiO2 rather than on a more physiological PaO2/FiO2 ratio. Hayashi and colleagues retrospectively analyzed the data from 543 patients receiving mechanical ventilation, comparing 153 patients with VAC to 390 without, and observed that those with VAC had a higher ICU length of stay (22 vs. 11 days), duration of mechanical ventilation (20 vs. 5 days), and use of antibiotics but no difference in overall ICU mortality [40]. VAC definitions were not specific for VAP and included atelectasis in 16.3 %, acute pulmonary edema in 11.8 %, and acute respiratory distress syndrome in 6.5 %. Thus the VAC definition identified sick patients, but not all VAC patients had pneumonia.
Muscedere and associates studied the clinical impact and preventability of VAC and IVAC, and the relationship to VAP, using prospectively collected data [41]. Of the 1,320 patients included, VAC developed in 10.5 % and patients who had VAC were more likely to develop VAP than those who did not (28.1 vs. 9.2 %, p < 0.001). However, only 39 of 139 with VAC had VAP, and most patients with VAP did not have VAC or IVAC. When prevention efforts were undertaken, they were able to reduce the incidence of VAC and VAP, but not IVAC. In another recent study of 2,080 patients from the Netherlands, electronic surveillance for VAE found the incidence of VAC, IVAC, and VAE-VAP to be 10.0, 4.2, and 3.2 per 1,000 ventilation days, respectively [42]. The incidence of VAE was higher in patients receiving at least 7 days of mechanical ventilation compared to those receiving less than 7 days. Only 32 % of patients with VAP identified by prospective surveillance were detected using the VAE algorithm.
Data from the above studies indicate that VAC and IVAC are not always VAP, and that prevention efforts for VAP do not always affect VAC and IVAC rates, raising doubt about the utility of these new definitions as a measure of the quality of care for ventilated patients.
Treatment of VAP
Therapy choices for VAP are based on the presence of risk factors for infection with MDR pathogens and may be initially broad-spectrum, necessitating de-escalation of therapy once clinical and microbiologic data become available. This approach balances the need for early appropriate therapy with avoidance of promoting antimicrobial resistance and drug toxicity, by not continuing multidrug therapy longer than is needed (see Fig. 1) [43].
Is there a difference in VAP outcomes between guideline-concordant and guideline-discordant therapy?
Ferrer and colleagues performed a validation study of the 2005 ATS guidelines in 276 patients with ICU-acquired pneumonia. Empiric treatment was considered adequate when the isolated pathogens were susceptible to one of the antimicrobials administrated, except for P. aeruginosa, where a combination of two active antibiotics was needed. Guideline adherence resulted more often in adequate therapy, compared to non-adherence (83 vs. 64 %, p = 0.013) [13]. In another multicenter trial with 740 mechanically ventilated patients, combination therapy with meropenem and ciprofloxacin increased the likelihood of appropriate empiric therapy in VAP for those with MDR pathogens (84.2 vs. 18.8 %, p < 0.001), but had no impact on mortality; however, in this population, MDR pathogens were uncommon [44]. In a study with initial empiric antibiotic therapy based on the German PEG guidelines, clinical improvement occurred in 82 % of the guideline-adherent group compared to 47 % in the non-adherent group (p = 0.001), with better survival in the guideline-adherent group (86 vs. 74 %, p = 0.021) [45].
Piskin and colleagues reported that previous antibiotic usage within 3 months (OR = 3.16) and admission to a surgical unit (OR = 2.9) were independent risk factors for inadequate initial VAP therapy [46]. In a study of 110 patients with ICU-acquired P. aeruginosa pneumonia, including 71 VAP patients, inadequate initial antibiotic therapy was related to higher mortality and more days on ventilation, and dual therapy was associated with less inadequate treatment [47]. In another multicenter study, using a propensity-matched cohort design, Kumar and colleagues evaluated 1,223 matched pairs of patients with septic shock, who received either adequate combination therapy or adequate monotherapy [16]. They found that early combination therapy with antibiotics having different mechanisms of action resulted in lower mortality (29 vs. 36.3 %; hazard ratio 0.77; 95 % confidence interval 0.67–0.88; p = 0.0002) than monotherapy, even though both groups of patients were receiving appropriate therapy. Also patients receiving combination therapy had increased ventilator and inotrope-free days (10 vs. 17; p = 0.008 and 23 vs. 25; p = 0.007) compared to patients receiving monotherapy.
In contrast to the data favoring the use of guideline-concordant, combination therapy, Kett and associates reported increased mortality when patients received therapy according to guidelines [7]. In a multicenter study of 303 patients with nosocomial pneumonia (132 VAP and 171 HAP + HCAP), patients who were not compliant with the ATS guidelines (n = 174), because of the non-use of dual coverage for Gram-negative pathogens or MRSA coverage, had a lower 28-day mortality (20 vs. 34 %), compared to guideline-complaint patients (n = 129). However, in this retrospective study, the reasons for patients receiving non-compliant regimens were not explained, and patients in the compliant arm were sicker (with higher APACHE II scores, and more often had sepsis), with less de-escalation of antibiotics, than the non-compliant group.
The focus on guideline adherence should not only be on starting a specific combination regimen but also ensuring that treatment is given as soon as possible, in proper dosages, on the basis of an updated local antibiogram, preferably with a different agent than used recently [48].
How does de-escalation of antibiotics affect VAP outcomes?
The IDSA antimicrobial stewardship guidelines emphasize antibiotic de-escalation as a method to optimize clinical outcomes, while avoiding antibiotic overuse, giving high evidence grading to this approach [49]. Other recommended stewardship approaches include prospective audit and physician feedback on antibiotic usage patterns, dose optimization, and the use of antibiotic treatment guidelines based on knowledge of local microbiologic patterns. While the benefits of de-escalation are becoming clear, there is much opportunity to increase its implementation, with the rates of de-escalation in VAP patients varying from 22 to 74 %. The highest rates occur when there is a protocol for antibiotic modification, rather than usual care, if initial therapy is appropriate, if cultures are positive rather than negative, and if the frequency of MDR pathogens is low [50].
Some studies have shown improved clinical outcomes for VAP when de-escalation is done. In one study of 137 patients with ICU-acquired pneumonia, de-escalation was done in 32 %, and these patients had a lower pneumonia-related mortality than those without de-escalation [51]. However, de-escalation may not have been the reason for a better outcome, but simply a marker of a responding patient, since those with de-escalation had a lower APACHE II and CPIS score at day 5 than those who did not. In a recent study of 714 patients with septic shock, the 35 % who had de-escalation had a lower mortality than those who did not, and this benefit persisted even if only those receiving appropriate therapy were examined [52]. In another observational study, including 89 suspected VAP patients with negative BAL results, investigators compared the effects of early discontinuation (antibiotics stopped within 1 day of final negative quantitative BAL culture results) to late discontinuation of antibiotics, and found no mortality benefit to prolonged therapy, with less frequent superinfections among those with early discontinuation [53].
Role of surveillance culture for de-escalation
One method to choose accurate, focused therapy for VAP is to base therapy choices on surveillance cultures of tracheal aspirates, collected before the onset of pneumonia. Luna and associates prospectively studied an antibiotic strategy based on routine endotracheal aspirate (ETA) culture in 283 ventilated patients, compared to empiric therapy based on the 2005 ATS/IDSA guidelines [54]. Sensitivity for ETA to predict a BAL-obtained pathogen was 62.4 % and was better if done within 3 days of VAP onset. Antibiotic choice based on the ATS/IDSA guidelines led to appropriate therapy in 97.9 %, compared to only 77.4 % based on ETA cultures, but there were fewer antibiotic days using the ETA-based culture. In another study, Brusselaers and colleagues performed a meta-analysis of 14 studies with 791 VAP episodes to analyze the predictive accuracy of twice weekly lower respiratory tract surveillance cultures on subsequent VAP microbiology [55]. The pooled sensitivity and specificity were 0.75 (95 % CI = 0.65–0.83) and 0.92 (95 % CI = 0.85–0.96) respectively for surveillance cultures, and the accuracy improved with twice weekly cultures and if only the latest cultures are used for prediction. But, most importantly, the surveillance cultures had a high negative likelihood value in culture-positive VAP patients especially with MDR pathogens and thus, potentially, routine surveillance cultures can reduce the antibiotic usage. However, the included studies in this meta-analysis are heterogeneous (included studies ranged from 1995 to 2012) and potentially subject to ascertainment bias. Thus, surveillance-based cultures may be helpful in guiding de-escalation and in reducing antibiotic use.
Biomarkers for de-escalation
Serial measurements of serum PCT have been shown to be able to reduce the duration of antibiotic therapy for ICU infection, without an adverse affect on outcome. Bouadma and colleagues performed a prospective, multicenter, open label trial in ICU infection, and found that with PCT guidance, patients with ICU infection (including 75 patients with VAP) had similar mortality as a control group (with duration of therapy based on guidelines), but with fewer days on antibiotics (absolute difference of 2.7 days) [56]. In another trial of 101 VAP patients randomized to antibiotic discontinuation according to guidelines (control group) versus serum PCT measurements, patients in the PCT group more “antibiotic free-days alive” at 28 days after VAP onset (13 vs. 9.5 days) compared to controls, and there was a 27 % reduction in overall antibiotic duration, with no difference in mortality, length of ICU stay, or days on mechanical ventilation [57].
Duration of treatment: short versus long
Chastre and colleagues established the safety and efficacy of 8 days’ duration of therapy for VAP, provided that patients received initially appropriate therapy and were not infected with non-fermenting Gram-negatives [58]. A subsequent study corroborated this finding and confirmed that using 15 versus 8 days of therapy led to no difference in mortality, ICU length of stay, and mechanical ventilation days [59]. However, the rate of secondary infection was higher in the shorter duration group (35.3 vs. 19.3 %, p = 0.01). In a recent multicenter, randomized, double-blind study of VAP, a 7-day course of high dose (1 g) of doripenem, given by a 4-h infusion, was compared to a 10-day course of a 1-g dose of imipenem, each given every 8 h [60]. The study was prematurely stopped because of documented inferiority of the 7-day regimen, particularly for VAP patients infected by P. aeruginosa. All-cause 28-day mortality was also higher in the doripenem arm compared to imipenem, although not significantly (21.5 vs. 14.8 %), but mortality was statistically higher for patients with P. aeruginosa who were treated with a 7-day course of doripenem. A recent meta-analysis of four randomized controlled trials comparing short (7–8 days) with longer (10–15 days) duration regimens in VAP showed no difference in mortality, and increased antibiotic-free days in the shorter course group [61]. There was a trend towards more relapses with non-fermenting Gram-negative bacilli in the shorter duration antibiotic cohort.
Other issues in VAP treatment
Although a comprehensive discussion is beyond the scope of this review, there are other areas of VAP pathogenicity and treatment that warrant attention (see Table 6).
Linezolid versus glycopeptides in the treatment of MRSA VAP
Choosing between the agents with activity against MRSA—linezolid, vancomycin, telavancin, or teicoplanin—has been controversial. Linezolid achieves higher lung epithelial lining fluid concentrations than glycopeptides and could therefore have an advantage for MRSA lung infections. However, two older meta-analyses demonstrated comparable clinical and microbiologic success with using linezolid and glycopeptides and a significantly increased risk of thrombocytopenia and gastrointestinal events with linezolid [62, 63]. Since those analyses, a multicenter prospective randomized trial of linezolid versus vancomycin for patients with documented MRSA pneumonia was completed, and linezolid led to a significantly higher rate of clinical response than optimally dosed vancomycin [64]. Although there was no difference in mortality between the two groups, this could potentially be explained by the fact that patients who failed vancomycin were able to be salvaged with linezolid, and any survival in this setting was attributed to vancomycin. Kalil and associates repeated their meta-analysis, using nine randomized controlled trials, including patients from the last study, and concluded that there was no significant difference in mortality and microbiological response comparing linezolid and vancomycin [65]. However, there were concerns regarding the quality of trials included in the analysis, with a trend in favor of linezolid in the better designed trials. In a response to this meta-analysis, Wunderink et al. found a significantly better clinical response with linezolid, if only patients with documented MRSA pneumonia were included [66]. Another recent meta-analysis that also included the new randomized controlled trial of linezolid versus vancomycin did find a higher microbiologic eradication rate for linezolid, and more nephrotoxicity when glycopeptides (vancomycin or teicoplanin) were used [67]. Recent data from the IMPACT-HAP study also demonstrated that patients with MRSA VAP treated with linezolid were more likely to experience clinical resolution of pneumonia than vancomycin-treated patients (p = 0.018) [68].
Role of tigecycline in treatment of VAP patients with A. baumannii
In a large double-blind, randomized, multicenter trial comparing imipenem/cilastatin to tigecycline in 945 HAP patients, those patients with VAP who were treated with tigecycline had a significantly lower cure rate and more deaths compared to patients with imipenem [69]. Therefore, several investigators have warned against using tigecycline monotherapy in VAP patients. Guner and associates reported the efficacy of tigecycline in 33 patients (19 with VAP) with carbapenem-resistant Acinetobacter spp., usually when used as part of a combination regimen [70]. In an exploratory study that tried to improve the efficacy of tigecycline against Acinetobacter spp. by increasing the dosing, tigecycline was comparable to imipenem in VAP patients, when used with a 200-mg loading dose, followed by 100 mg every 12 h (which is twice the dose that was used in the negative study above) [71]. Montravers and associates in a prospective study of 156 ICU patients treated with tigecycline (mostly intra-abdominal infections and 24 % lung infections) noted that the success rate was higher with longer duration of treatment (more than 9 days). They used tigecycline as part of a combination regimen in 67 % of patients, but with no impact of combination therapy on mortality [72].
Use of adjunctive inhaled antibiotics for the therapy of MDR pathogens
The use of adjunctive aerosolized antibiotics such as aminoglycosides, colistin, or ceftriazone can achieve high concentrations in the lung that could overcome in vitro levels of resistance, and also avoid the poor lung penetration of certain systemic antibiotics. This may be helpful in VAP patients who are not responding despite adequate systemic therapy or in patients infected with highly resistant organisms (Fig. 2). In a prospective randomized controlled trial, use of adjunctive aerosolized amikacin, compared to placebo, and combined with systemic antibiotics, the aerosol therapy led to a shorter duration of systemic therapy. The study was done using an investigational drug-delivery system in patients with Gram-negative VAP and at risk for MDR pathogens [73]. In another randomized study, patients received aerosolized ceftazidime and amikacin as the only agent for treatment of P. aeruginosa VAP, compared to systemic therapy and after 8 days of treatment, both the systemic therapy group and the inhalation therapy group had similar efficacy, treatment failure, and super-infection rates [74].
a, b CT scan of the chest of a 68-year-old woman with history of breast cancer on chemotherapy, diagnosed with Gram-negative ventilator-associated pneumonia (bronchoscopy with biopsy and BAL—positive for Klebsiella in culture and in the tissues, along with E. coli). She was initially treated with tigecycline in view of beta-lactam allergy but had clinical and radiographic deterioration (b). Antibiotics were changed to intravenous aztreonam and inhaled tobramycin with clinical and radiographic improvement
Continuous/prolonged infusion of antibiotics in VAP
Pharmacokinetics and pharmacodynamics are altered in patients with severe sepsis and dose adjustment may be necessary. Bactericidal activity related with aminoglycosides and quinolones is concentration-dependent, while with the beta-lactams, killing is dependent on how long the antibiotic concentration exceeds the minimum inhibitory concentration (MIC) of the target pathogen, and killing can be optimized by prolonged or continuous infusion methods. Nicasio and associates used an optimized dosing clinical pathway (incorporating prolonged infusions of cefepime and meropenem) and observed a reduction of infection-related mortality in 94 patients with VAP [75]. Dulhunty and colleagues in a double-blind randomized controlled trial compared continuous versus intermittent bolus dosing of piperacillin–tazobactam, meropenem, and ticarcillin–clavulanate in 30 patients with severe sepsis and found higher concentration above MIC and higher clinical cure rate, but no difference in ICU or hospital length of stay or mortality in the intervention arm than in the 30 control subjects [76].
VAP in trauma patients
Trauma patients have a higher incidence of VAP that is associated with prolonged mechanical ventilation and late tracheostomy. In a prospective, multicenter, observational study of 630 trauma patients, predictors of VAP were male sex and pulmonary contusion, while using a ‘VAP prevention bundle’ did not reduce the VAP incidence. Mortality in this study was related to age and higher APACHE II scores and injury severity scores [77]. Chaari and associates in a prospective study showed that late tracheotomy and stomal infection were independent factors predicting VAP onset, and patients with VAP had prolonged mechanical ventilation and ICU stay, but not an increased overall mortality [78]. Parks et al. in a study of 1,028 VAP episodes in trauma patients noted that using CPIS scores would have resulted in unnecessary antibiotic continuation in 59 %. CPIS has low sensitivity and specificity in determining VAP resolution in trauma patients [79]. Trauma patients with Glasgow coma score less than 9 are at risk for staphylococcal infection. In a study comparing intermittent versus continuous infusion of vancomycin in trauma patients with suspected VAP, more patients in the continuous group achieved therapeutic concentrations (7.4 vs. 57.1 %, p < 0.0001) with a similar incidence of adverse renal injury [80].
Prevention
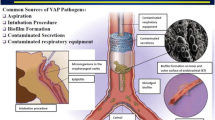
VAP prevention measures are increasingly being adopted as a quality of care indicator in ICUs around the world. Endotracheal tube biofilm formation and microaspiration of oropharyngeal secretions, contaminated by endogenous flora, remain as important mechanisms for the development of VAP and are the target of several intervention strategies. In many ICUs, prevention strategies have been implemented in the form of “ventilator bundles”, which have been able to reduce VAP rates, but controversy remains about whether it is possible to reduce rates to achieve “zero VAP”. Ventilator bundles commonly include daily interruption of sedation, daily weaning trials, head of the bed elevation, and oral care. In addition, selected ICUs have added newer endotracheal tubes (silver coated, or with modified cuffs to avoid aspiration), subglottic secretion drainage via continuous or intermittent suction, new devices to remove biofilm from the inside of the endotracheal tube, saline instillation prior to suction, and early tracheostomy [81].
What is the role of VAP prevention bundles and behavior modification strategies in VAP incidence?
A group of ventilator care strategies, when performed as part of a daily care bundle, have been shown to decrease the VAP rates [82]. In a study from Scotland analyzing the effects of a VAP prevention bundle, there was a significant reduction in the reported VAP rates, post intervention, compared to the pre-intervention time period (12 vs. 32/1,000 ventilator days, p < 0.001), but no difference in mechanical ventilation days or ICU length of stay [8]. Despite several behavior modification strategies, including the use of nurse and medical champions, teaching materials, education sessions, bedside cues, and feedback about compliance, the overall bundle compliance rate was 70 % in this study. In another multicenter study, involving 1,320 patients, investigators used a 2-year multifaceted intervention via educational sessions, supplemented with reminders and local opinion leaders, to improve concordance with VAP prevention and treatment guidelines [83]. Over time, there was more improvement in prevention strategies than in therapy approaches, and overall, a significant increase in guideline concordance [aggregate concordance (mean [SD]), 50.7 % (6.1), 54.4 % (7.1), 56.2 % (5.9), 58.7 % (6.7) p = 0.007, in three consecutive 6-month periods]. They also observed a reduction in VAP rates (events/330 patients): 47 (14.2 %), 34 (10.3 %), 38(11.5 %), 29 (8.8 %) (p = 0.03) over the study period, but ICU mortality and length of ICU remained unchanged. Thus the data from these two studies showed a decrease in VAP rates after introduction of preventive interventions, but also highlight several potential barriers to guideline implementation and variable practices that exist within the community.
Is there a role of early tracheostomy to avoid VAP development?
Studies of early tracheostomy and its influence in reducing the incidence of VAP have not been conclusive, with some studies showing benefit, while others with no clear advantage. A meta-analysis by Griffiths and colleagues compared early tracheostomy with either late tracheostomy or prolonged endotracheal intubation. Early tracheostomy (within 7 days of invasive mechanical ventilation) did not significantly reduce the risk of VAP or mortality but reduced the number of days on the ventilator and ICU stay [84]. Blot and associates randomized critically ill patients expected to require more than 7 days of mechanical ventilation to either (open or percutaneous) early tracheostomy within 4 days or prolonged intubation [85]. The study was prematurely stopped after enrolling 125 patients as no difference was found between the two groups in mortality, VAP incidence, duration of mechanical ventilation, ICU stay, sedation use, or laryngeal or tracheal complications. In another trial, investigators compared the effectiveness of early tracheotomy (after 6–8 days of endotracheal intubation) to late tracheotomy (after 13–15 days) in reducing the incidence of pneumonia [86]. Even though 209 patients were randomized to the early group and 210 patients to the late group, 31 % of the early group and 43 % of the late group did not receive tracheostomy. Although there was a trend towards decreased VAP incidence with the early tracheotomy group, there was no significant difference between the groups in VAP rates (14 vs. 21 %, p = 0.07) or mortality. As with earlier trials, there were significantly more ventilator-free days and ICU-free days at 28 days in the early tracheotomy group. Another recent multicenter, open, randomized trial by Young and associates did not show any difference in 30-day mortality, length of ICU stay, or tracheostomy-related complications in the early (within 4 days) compared to late tracheostomy group (after 10 days) [87]. Thus on the basis of these studies there is not enough compelling evidence that routine early tracheotomy can prevent the development of pneumonia, but patients who underwent early tracheostomy had significantly more ventilator-free days.
Conclusion
VAP microbial etiology has changed over the last several decades and the relative prevalence of individual pathogens varies based on geographic location and patient risk factors. The morbidity and mortality related to VAP remain high and, in the absence of a gold standard test for diagnosis, suspected VAP patients should be started on antibiotics recommended as per the 2005 ATS guidelines and a knowledge of local antibiotic susceptibility patterns. Using a combination of clinical severity scores, biomarkers, and cultures might help with reducing the duration of therapy and achieving antibiotic de-escalation. The new CDC surveillance definition of VAE is not helpful in identifying all patients with VAP. There is still uncertainty regarding VAP diagnosis and management, and controversies are highlighted in this review. Having a centralized national registry for VAP patients might be beneficial in answering several of the controversial questions, but governmental penalties and institutional policies might be challenging as we seek to achieve this goal.
References
Niederman MS, Craven D, Bonten MJ, Chastre J, Craig W, Fagon J, Hall J, Jacoby G, Kollef M, Luna C, Mandell L, Torres A, Wunderink R (2005) Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 171:388–416
Dudeck MA, Horan TC, Peterson KD, Allen-Bridson K, Morrell GC, Pollock DA, Edwards JR (2011) National Healthcare Safety Network (NHSN) report, data summary for 2009, device-associated module. Am J Infect Control 39:349–367
Klompas M (2012) Is a ventilator-associated pneumonia rate of zero really possible? Curr Opin Infect Dis 25:176–182
Klompas M, Magill S, Robicsek A, Strymish JM, Kleinman K, Evans RS, Lloyd JF, Khan Y, Yokoe DS, Stevenson K, Samore M, Platt R (2012) Objective surveillance definitions for ventilator-associated pneumonia. Crit Care Med 40:3154–3161
Heyland D, Cook D, Dodek P, Muscedere J, Day A (2006) A randomized trial of diagnostic techniques for ventilator-associated pneumonia. N Engl J Med 355:2619–2630
Aarts MA, Hancock JN, Heyland D, McLeod RS, Marshall JC (2008) Empiric antibiotic therapy for suspected ventilator-associated pneumonia: a systematic review and meta-analysis of randomized trials. Crit Care Med 36:108–117
Kett DH, Cano E, Quartin AA, Mangino JE, Zervos MJ, Peyrani P, Cely CM, Ford KD, Scerpella EG, Ramirez JA (2011) Implementation of guidelines for management of possible multidrug-resistant pneumonia in intensive care: an observational, multicentre cohort study. Lancet Infect Dis 11:181–189
Morris AC, Hay AW, Swann DG, Everingham K, McCulloch C, McNulty J, Brooks O, Laurenson IF, Cook B, Walsh TS (2011) Reducing ventilator-associated pneumonia in intensive care: impact of implementing a care bundle. Crit Care Med 39:2218–2224
Giantsou E, Liratzopoulos N, Efraimidou E, Panopoulou M, Alepopoulou E, Kartali-Ktenidou S, Minopoulos GI, Zakynthinos S, Manolas KI (2005) Both early-onset and late-onset ventilator-associated pneumonia are caused mainly by potentially multiresistant bacteria. Intensive Care Med 31:1488–1494
Muscedere J, Dodek P, Keenan S, Fowler R, Cook D, Heyland D (2008) Comprehensive evidence-based clinical practice guidelines for ventilator-associated pneumonia: diagnosis and treatment. J Crit Care 23:138–147
Gastmeier P, Sohr D, Geffers C, Rüden H, Vonberg RP, Welte T (2009) Early- and late-onset pneumonia: is this still a useful classification? Antimicrob Agents Chemother 53:2714–2718
Restrepo M, Peterson J, Fernandez JF, Qin Z, Fisher AC, Nicholson SC (2013) Comparison of the bacterial etiology of early-onset and late-onset ventilator-associated pneumonia in subjects enrolled in 2 large clinical studies. Respir Care 58:1220–1225
Ferrer M, Liapikou A, Valencia M, Esperatti M, Theessen A, Antonio Martinez J, Mensa J, Torres A (2010) Validation of the American Thoracic Society-Infectious Diseases Society of America guidelines for hospital-acquired pneumonia in the intensive care unit. Clin Infect Dis 50:945–952
Martin-Loeches Deja M, Koulenti D, Dimopoulos G, Marsh B, Torres A, Niederman MS, Rello J (2013) Potentially resistant microorganisms in intubated patients with hospital-acquired pneumonia: the interaction of ecology, shock and risk factors. Intensive Care Med 39:672–681
Iregui M, Ward S, Sherman G, Fraser VJ, Kollef MH (2002) Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator-associated pneumonia. Chest 122:262–268
Kumar A, Zarychanski R, Light B, Parrillo J, Maki D, Simon D, Laporta D, Lapinsky S, Ellis P, Mirzanejad Y, Martinka G, Keenan S, Wood G, Arabi Y, Feinstein D, Kumar A, Dodek P, Kravetsky L, Doucette S (2010) Early combination antibiotic therapy yields improved survival compared with monotherapy in septic shock: a propensity-matched analysis. Crit Care Med 38:1773–1785
Kollef MH (2000) Inadequate antimicrobial treatment: an important determinant of outcome for hospitalized patients. Clin Infect Dis 31(Suppl 4):S131–S138
Jones RN (2010) Microbial etiologies of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clin Infect Dis 50:S81–S87
Esperatti M, Ferrer M, Theessen A, Liapikou A, Valencia M, Saucedo LM, Zavala E, Welte T, Torres A (2010) Nosocomial pneumonia in the intensive care unit acquired by mechanically ventilated versus nonventilated patients. Am J Respir Crit Care Med 182:1533–1539
Weber DJ, Rutala WA, Sickbert-Bennett EE, Samsa GP, Brown V, Niederman MS (2007) Microbiology of ventilator-associated pneumonia compared with that of hospital-acquired pneumonia. Infect Control Hosp Epidemiol 28:825–831
Horan TC, Andrus M, Dudeck MA (2008) CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 36:309–332
Chastre J, Fagon JY (2002) Ventilator-associated pneumonia. Am J Respir Crit Care Med 165:867–903
Pugin J, Auckenthaler R, Mili N, Janssens JP, Lew PD, Suter PM (1991) Diagnosis of ventilator-associated pneumonia by bacteriologic analysis of bronchoscopic and nonbronchoscopic “blind” bronchoalveolar lavage fluid. Am Rev Respir Dis 143:1121–1129
Singh N, Rogers P, Atwood CW, Wagener MM, Yu VL (2000) Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med 162:505–511
Craven DE, Chroneou A, Zias N, Hjalmarson KI (2009) Ventilator-associated tracheobronchitis: the impact of targeted antibiotic therapy on patient outcomes. Chest 135:521–528
Nseir S, Favory R, Jozefowicz E, Decamps F, Dewavrin F, Brunin G, Di Pompeo C, Mathieu D, Durocher A (2008) Antimicrobial treatment for ventilator-associated tracheobronchitis: a randomized, controlled, multicenter study. Crit Care 12:R62
Dallas J, Skrupky L, Abebe N, Boyle WA 3rd, Kollef MH (2011) Ventilator-associated tracheobronchitis in a mixed surgical and medical ICU population. Chest 139:513–518
Craven DE, Hjalmarson K (2008) Prophylaxis of ventilator-associated pneumonia: changing culture and strategies to trump disease. Chest 134:898–900
Shorr AF, Sherner JH, Jackson WL, Kollef MH (2005) Invasive approaches to the diagnosis of ventilator-associated pneumonia: a meta-analysis. Crit Care Med 33:46–53
Berton DC, Kalil AC, Teixeira PJ (2012) Quantitative versus qualitative cultures of respiratory secretions for clinical outcomes in patients with ventilator-associated pneumonia. Cochrane Database Syst Rev 1:CD006482
Zilberberg MD, Shorr AF (2010) Ventilator-associated pneumonia: the clinical pulmonary infection score as a surrogate for diagnostics and outcome. Clin Infect Dis 51:S131–S135
Luyt CE, Chastre J, Fagon JY (2004) Value of the clinical pulmonary infection score for the identification and management of ventilator-associated pneumonia. Intensive Care Med 30:844–852
Michel F, Franceschini B, Berger P, Arnal JM, Gainnier M, Sainty JM, Papazian L (2005) Early antibiotic treatment for BAL-confirmed ventilator-associated pneumonia: a role for routine endotracheal aspirate cultures. Chest 127:589–597
Luna CM, Blanzaco D, Niederman MS, Matarucco W, Baredes NC, Desmery P, Palizas F, Menga G, Rios F, Apezteguia C (2003) Resolution of ventilator-associated pneumonia: prospective evaluation of the clinical pulmonary infection score as an early clinical predictor of outcome. Crit Care Med 31:676–682
Luyt CE, Guérin V, Combes A, Trouillet JL, Ayed SB, Bernard M, Gibert C, Chastre J (2005) Procalcitonin kinetics as a prognostic marker of ventilator-associated pneumonia. Am J Respir Crit Care Med 171:48–53
Ramirez P, Garcia MA, Ferrer M, Aznar J, Valencia M, Sahuquillo JM, Menéndez R, Asenjo MA, Torres A (2008) Sequential measurements of procalcitonin levels in diagnosing ventilator-associated pneumonia. Eur Respir J 31:356–362
Klompas M, Kleinman K, Khan Y, Evans RS, Lloyd JF, Stevenson K, Samore M, Platt R (2012) Rapid and reproducible surveillance for ventilator-associated pneumonia. Clin Infect Dis 54:370–377
Klompas M, Khan Y, Kleinman K, Evans RS, Lloyd JF, Stevenson K, Samore M, Platt R (2011) Multicenter evaluation of a novel surveillance paradigm for complications of mechanical ventilation. PLoS One 6:e18062
Magill SS, Klompas M, Balk R, Burns SM, Deutschman CS, Diekema D, Fridkin S, Greene L, Guh A, Gutterman D, Hammer B, Henderson D, Hess DR, Hill NS, Horan T, Kollef M, Levy M, Septimus E, Vanantwerpen C, Wright D, Lipsett P (2013) Executive summary: developing a new, national approach to surveillance for ventilator-associated events. Ann Am Thorac Soc 10:S220–S223
Hayashi Y, Morisawa K, Klompas M, Jones M, Bandeshe H, Boots R, Lipman J, Paterson DL (2013) Toward improved surveillance: the impact of ventilator-associated complications on length of stay and antibiotic use in patients in intensive care units. Clin Infect Dis 56:471–477
Muscedere J, Sinuff T, Heyland DK, Dodek PM, Keenan SP, Wood G, Jiang X, Day AG, Laporta D, Klompas M (2013) The clinical impact and preventability of ventilator-associated conditions in critically ill patients who are mechanically ventilated. Chest 144:1453–1460
Klein Klouwenberg PM, van Mourik MS, Ong DS, Horn J, Schultz MJ, Cremer OL, Bonten MJ (2014) Electronic implementation of a novel surveillance paradigm for ventilator-associated events: feasibility and validation. Am J Respir Crit Care Med 189:947–955
Kollef MH (2001) Hospital-acquired pneumonia and de-escalation of antimicrobial treatment. Crit Care Med 29:1473–1475
Heyland DK, Dodek P, Muscedere J, Day A, Cook D (2008) Randomized trial of combination versus monotherapy for the empiric treatment of suspected ventilator-associated pneumonia. Crit Care Med 36:737–744
Wilke M, Grube RF, Bodmann KF (2011) Guideline-adherent initial intravenous antibiotic therapy for hospital-acquired/ventilator-associated pneumonia is clinically superior, saves lives and is cheaper than non guideline adherent therapy. Eur J Med Res 16:315–323
Piskin N, Aydemir H, Oztoprak N, Akduman D, Comert F, Kokturk F, Celebi G (2012) Inadequate treatment of ventilator-associated and hospital-acquired pneumonia: risk factors and impact on outcomes. BMC Infect Dis 12:268
Tumbarello M, De Pascale G, Trecarichi EM, Spanu T, Antonicelli F, Maviglia R, Pennisi MA, Bello G, Antonelli M (2013) Clinical outcomes of Pseudomonas aeruginosa pneumonia in intensive care unit patients. Intensive Care Med 39:682–692
Trouillet JL, Vuagnat A, Combes A, Kassis N, Chastre J, Gibert C (2002) Pseudomonas aeruginosa ventilator-associated pneumonia: comparison of episodes due to piperacillin-resistant versus piperacillin-susceptible organisms. Clin Infect Dis 34:1047–1054
Dellit TH, Owens RC, McGowan JE Jr, Gerding DN, Weinstein RA, Burke JP, Huskins WC, Paterson DL, Fishman NO, Carpenter CF, Brennan PJ, Billeter M, Hooton TM (2007) Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis 44:159–177
Niederman MS, Soulountsi V (2011) De-escalation therapy: is it valuable for the management of ventilator-associated pneumonia? Clin Chest Med 32:517–534
Joung MK, Lee JA, Moon SY, Cheong HS, Joo EJ, Ha YE, Sohn KM, Chung SM, Suh GY, Chung DR, Song JH, Peck KR (2011) Impact of de-escalation therapy on clinical outcomes for intensive care unit-acquired pneumonia. Crit Care 15:R79
Garnacho-Montero J, Gutiérrez-Pizarraya A, Escoresca-Ortega A, Corcia-Palomo Y, Fernández-Delgado E, Herrera-Melero I, Ortiz-Leyba C, Márquez-Vácaro JA (2014) De-escalation of empirical therapy is associated with lower mortality in patients with severe sepsis and septic shock. Intensive Care Med 40:32–40
Raman K, Nailor MD, Nicolau DP, Aslanzadeh J, Nadeau M, Kuti JL (2013) Early antibiotic discontinuation in patients with clinically suspected ventilator-associated pneumonia and negative quantitative bronchoscopy cultures. Crit Care Med 41:1656–1663
Luna CM, Sarquis S, Niederman MS, Sosa FA, Otaola M, Bailleau N, Vay CA, Famiglietti A, Irrazabal C, Capdevila AA (2013) Is a strategy based on routine endotracheal cultures the best way to prescribe antibiotics in ventilator-associated pneumonia? Chest 144:63–71
Brusselaers N, Labeau S, Vogelaers D, Blot S (2013) Value of lower respiratory tract surveillance cultures to predict bacterial pathogens in ventilator-associated pneumonia: systematic review and diagnostic test accuracy meta-analysis. Intensive Care Med 39:365–375
Bouadma L, Luyt CE, Tubach F, Cracco C, Alvarez A, Schwebel C, Schortgen F, Lasocki S, Veber B, Dehoux M, Bernard M, Pasquet B, Régnier B, Brun-Buisson C, Chastre J, Wolff M (2010) Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet 375:463–474
Stolz D, Smyrnios N, Eggimann P, Pargger H, Thakkar N, Siegemund M, Marsch S, Azzola A, Rakic J, Mueller B, Tamm M (2009) Procalcitonin for reduced antibiotic exposure in ventilator-associated pneumonia: a randomised study. Eur Respir J 34:1364–1375
Chastre J, Wolff M, Fagon JY, Chevret S, Thomas F, Wermert D, Clementi E, Gonzalez J, Jusserand D, Asfar P, Perrin D, Fieux F, Aubas S (2003) Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA 290:2588–2598
Capellier G, Mockly H, Charpentier C, Annane D, Blasco G, Desmettre T, Roch A, Faisy C, Cousson J, Limat S, Mercier M, Papazian L (2012) Early-onset ventilator-associated pneumonia in adults randomized clinical trial: comparison of 8 versus 15 days of antibiotic treatment. PLoS One 7:e41290
Kollef MH, Chastre J, Clavel M, Restrepo MI, Michiels B, Kaniga K, Cirillo I, Kimko H, Redman R (2012) A randomized trial of 7-day doripenem versus 10-day imipenem-cilastatin for ventilator-associated pneumonia. Crit Care 16:R218
Dimopoulos G, Poulakou G, Pneumatikos IA, Armaganidis A, Kollef MH, Matthaiou DK (2013) Short- vs long-duration antibiotic regimens for ventilator-associated pneumonia: a systematic review and meta-analysis. Chest 144:1759–1767
Walkey AJ, O’Donnell MR, Wiener RS (2011) Linezolid vs glycopeptide antibiotics for the treatment of suspected methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a meta-analysis of randomized controlled trials. Chest 139:1148–1155
Kalil AC, Murthy MH, Hermsen ED, Neto FK, Sun J, Rupp ME (2010) Linezolid versus vancomycin or teicoplanin for nosocomial pneumonia: a systematic review and meta-analysis. Crit Care Med 38:1802–1808
Wunderink RG, Niederman MS, Kollef MH, Shorr AF, Kunkel MJ, Baruch A, McGee WT, Reisman A, Chastre J (2012) Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis 54:621–629
Kalil AC, Klompas M, Haynatzki G, Rupp ME (2013) Treatment of hospital-acquired pneumonia with linezolid or vancomycin: a systematic review and meta-analysis. BMJ Open 3:e003912
Wunderink RG, Shorr AF, Niederman MS, Kollef MH, McGee WT, Chastre J (2014) Which antibiotic for hospital acquired pneumonia caused by MRSA? http://www.bmj.com/content/348/bmj.g1469/rr/689916. Accessed 6 Apr 14
Jiang H, Tang RN, Wang J (2013) Linezolid versus vancomycin or teicoplanin for nosocomial pneumonia: meta-analysis of randomised controlled trials. Eur J Clin Microbiol Infect Dis 32:1121–1128
Peyrani P, Wiemken TL, Kelley R, Zervos MJ, Kett DH, File TM Jr, Stein GE, Ford KD, Scerpella EG, Welch V, Ramirez JA (2014) Higher clinical success in patients with ventilator-associated pneumonia due to methicillin-resistant Staphylococcus aureus treated with linezolid compared with vancomycin: results from the IMPACT-HAP study. Crit Care 18:R118
Freire AT, Melnyk V, Kim MJ, Datsenko O, Dzyublik O, Glumcher F, Chuang YC, Maroko RT, Dukart G, Cooper CA, Korth-Bradley JM, Dartois N, Gandjini H (2010) Comparison of tigecycline with imipenem/cilastatin for the treatment of hospital-acquired pneumonia. Diagn Microbiol Infect Dis 68:140–151
Guner R, Hasanoglu I, Keske S, Kalem AK, Tasyaran MA (2011) Outcomes in patients infected with carbapenem-resistant Acinetobacter baumannii and treated with tigecycline alone or in combination therapy. Infection 39:515–518
Ramirez J, Dartois N, Gandjini H, Yan JL, Korth-Bradley J, McGovern PC (2013) Randomized phase 2 trial to evaluate the clinical efficacy of two high-dosage tigecycline regimens versus imipenem-cilastatin for treatment of hospital-acquired pneumonia. Antimicrob Agents Chemother 57:1756–1762
Montravers P, Dupont H, Bedos JP, Bret P (2014) Tigecycline use in critically ill patients: a multicentre prospective observational study in the intensive care setting. Intensive Care Med 40:988–997
Niederman MS, Chastre J, Corkery K, Fink JB, Luyt CE, García MS (2012) BAY41-6551 achieves bactericidal tracheal aspirate amikacin concentrations in mechanically ventilated patients with Gram-negative pneumonia. Intensive Care Med 38:263–271
Lu Q, Yang J, Liu Z, Gutierrez C, Aymard G, Rouby JJ (2011) Nebulized ceftazidime and amikacin in ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Am J Respir Crit Care Med 184:106–115
Nicasio AM, Eagye KJ, Nicolau DP, Shore E, Palter M, Pepe J (2010) Pharmacodynamic-based clinical pathway for empiric antibiotic choice in patients with ventilator-associated pneumonia. J Crit Care 25:69–77
Dulhunty JM, Roberts JA, Davis JS, Webb SA, Bellomo R, Gomersall C, Shirwadkar C, Eastwood GM, Myburgh J, Paterson DL (2013) Continuous infusion of beta-lactam antibiotics in severe sepsis: a multicenter double-blind, randomized controlled trial. Clin Infect Disease 56:236–244
Croce MA, Brasel KJ, Coimbra R, Adams CA Jr, Miller PR, Pasquale MD, McDonald CS, Vuthipadadon S, Fabian TC, Tolley EA (2013) National Trauma Institute prospective evaluation of the ventilator bundle in trauma patients: does it really work? J Trauma Acute Care Surg 74:354–360
Chaari A, Kssibi H, Zribi W, Medhioub F, Chelly H, Algia NB, Hamida CB, Bahloul M, Bouaziz M (2013) Ventilator-associated pneumonia in trauma patients with open tracheotomy: predictive factors and prognosis impact. J Emerg Trauma Shock 6:246–251
Parks NA, Magnotti LJ, Weinberg JA, Zarzaur BL, Schroeppel TJ, Swanson JM, Fabian TC, Croce MA (2012) Use of the clinical pulmonary infection score to guide therapy for ventilator-associated pneumonia risks antibiotic overexposure in patients with trauma. J Trauma Acute Care Surg 73:52–58
Schmelzer TM, Christmas AB, Norton HJ, Heniford BT, Sing RF (2013) Vancomycin intermittent dosing versus continuous infusion for treatment of ventilator-associated pneumonia in trauma patients. Am Surg 79:1185–1190
Bouadma L, Wolff M, Lucet JC (2012) Ventilator-associated pneumonia and its prevention. Curr Opin Infect Dis 25:395–404
Resar R, Pronovost P, Haraden C, Simmonds T, Rainey T, Nolan T (2005) Using a bundle approach to improve ventilator care processes and reduce ventilator-associated pneumonia. Jt Comm J Qual Patient Saf 31:243–248
Sinuff T, Muscedere J, Cook DJ, Dodek PM, Anderson W, Keenan SP, Wood G, Tan R, Haupt MT, Miletin M, Bouali R, Jiang X, Day AG, Overvelde J, Heyland DK (2013) Implementation of clinical practice guidelines for ventilator-associated pneumonia: a multicenter prospective study. Crit Care Med 41:15–23
Griffiths J, Barber VS, Morgan L, Young JD (2005) Systematic review and meta-analysis of studies of the timing of tracheostomy in adult patients undergoing artificial ventilation. BMJ 330:1243
Blot F, Similowski T, Trouillet JL, Chardon P, Korach JM, Costa MA, Journois D, Thiéry G, Fartoukh M, Pipien I, Bruder N, Orlikowski D, Tankere F, Durand-Zaleski I, Auboyer C, Nitenberg G, Holzapfel L, Tenaillon A, Chastre J, Laplanche A (2008) Early tracheotomy versus prolonged endotracheal intubation in unselected severely ill ICU patients. Intensive Care Med 34:1779–1787
Terragni PP, Antonelli M, Fumagalli R, Faggiano C, Berardino M, Pallavicini FB, Miletto A, Mangione S, Sinardi AU, Pastorelli M, Vivaldi N, Pasetto A, Della Rocca G, Urbino R, Filippini C, Pagano E, Evangelista A, Ciccone G, Mascia L, Ranieri VM (2010) Early vs late tracheotomy for prevention of pneumonia in mechanically ventilated adult ICU patients: a randomized controlled trial. JAMA 303:1483–1489
Young D, Harrison DA, Cuthbertson BH, Rowan K (2013) Effect of early vs late tracheostomy placement on survival in patients receiving mechanical ventilation: the TracMan randomized trial. JAMA 309:2121–2129
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nair, G.B., Niederman, M.S. Ventilator-associated pneumonia: present understanding and ongoing debates. Intensive Care Med 41, 34–48 (2015). https://doi.org/10.1007/s00134-014-3564-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-014-3564-5