Abstract
Context
Pressure support ventilation (PSV) must be tailored to the load capacity balance of the respiratory system. While "over assistance" generated hyperinflation and ineffective efforts, "under assistance" increased respiratory drive and causes dyspnea. Surface electromyograms (sEMGs) of extradiaphragmatic inspiratory muscles were responsive to respiratory loading/unloading.
Objectives
To determine if sEMGs of extradiaphragmatic inspiratory muscles vary with PSV settings and relate to the degree of discomfort and the intensity of dyspnea in acutely ill patients.
Design
Pathophysiological study, prospective inclusions of 12 intubated adult patients.
Interventions
Two PSV levels (high and low) and two expiratory trigger (ET) levels (high and low).
Measurements
Surface electromyograms of the scalene, parasternal, and Alae Nasi muscles (peak, EMGmax; area under the curve, EMGAUC); dyspnea visual analogue scale (VAS); prevalence of ineffective triggering efforts.
Main results
For the three recorded muscles, EMGmax and EMGAUC were significantly greater with low PS than high PS. The influence of ET was less important. A strong correlation was found between dyspnea and EMGmax. A significant inverse correlation was found between the prevalence of ineffective efforts and both dyspnea-VAS and EMGmin.
Conclusions
Surface electromyograms of extradiaphragmatic inspiratory muscles provides a simple, reliable and non-invasive indicator of respiratory muscle loading/unloading in mechanically ventilated patients. Because this EMG activity is strongly correlated to the intensity of dyspnea, it could be used as a surrogate of respiratory sensations in mechanically ventilated patients, and might, therefore, provide a monitoring tool in patients in whom detection and quantification of dyspnea is complex if not impossible.
Similar content being viewed by others
Introduction
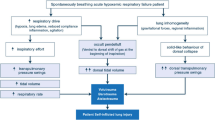
There is growing evidence that dyspnea or respiratory discomfort is a critical issue in mechanically ventilated patients. Indeed, up to half of them experience a substantial level of dyspnea [1], which is associated with negative clinical outcomes [1]. Inappropriate ventilator settings, most often leading to “under assistance”, are among the main determinants of dyspnea under mechanical ventilation [1, 2]. In such circumstances, dyspnea can be alleviated by increasing tidal volume (VT), but with the risk of “over assistance” that can be also be deleterious. Indeed, over assistance can induce dynamic hyperinflation and patient–ventilator asynchronies [3, 4], also associated with negative outcomes [4]. This justifies the search for monitoring tools that could help tailor ventilatory assistance to the demand of the patient without exceeding it (Fig. 1).
The electromyographic activity (EMG) of the extradiaphragmatic inspiratory muscles relates to the inspiratory drive to breathe [12, 13] and to respiratory sensations. It is therefore a logical monitoring target in the perspective of dynamically adapting ventilatory assistance. Surface EMGs of these muscles can be recorded in a routine ICU setting [5], but their value as surrogate biomarkers of dyspnea and their responsiveness to ventilator settings have seemingly not been studied in intubated, mechanically ventilated ICU patients.
In the present study, we hypothesised that the inspiratory electromyographic activity (iEMG) of three extradiaphragmatic inspiratory muscles (Alae nasi, scalenes, and parasternal intercostal) would vary with the level of ventilatory support administered to ICU patients and be related to the intensity of dyspnea if present. Additionally, we also studied the relationship between surface iEMGs, dyspnea, and the prevalence of ineffective inspiratory triggering efforts (a type of patient–ventilator asynchrony that is characteristic of over assistance).
Patients and methods
The study was conducted in a 16-bed ICU within a 1,600-bed university hospital. It was externally approved with regard to ethics and compliance to the French law on biomedical research (“Comité de protection des personnes—Ile de France VI). Informed consent was obtained from the patients.
Patients
Intubated or tracheostomized patients were eligible for inclusion in the study if: (1) they had been mechanically ventilated with inspiratory pressure support (IPS) for at least 12 h; (2) they had received no sedative, vasopressor or inotropic medication during the last 12 h; (3) their Ramsay score was ≤ 3 [6]; (4) according to the validated ATICE scale [7] they were awake and able to obey five commands (“open/close your eyes,” “look at me,” “open your mouth and put out your tongue,” “nod your head,” and “raise your eyebrows when I have counted up to five”). Patients were not included in the study when communication was likely to be difficult (auditory or visual impairment, insufficient command of French), when they were known to suffer from prior psychiatric or neurological disease, or when they presented with obvious delirium at the time of evaluation.
The study pertains to a convenience sample of twelve patients (Table 1).
Study protocol
The patients were ventilated using a Servo-i ventilator (Maquet Critical Care, Solna, Sweden). Positive end expiratory pressure (PEEP) was set at 4 cmH2O and the fractional concentration of oxygen (FiO2) was set to achieve a SpO2 of 92 to 96 %. Two levels of pressure support (PS) were sequentially applied in random order. A low PS level (Low PS) targeted a VT of 6–8 ml/kg whereas a high PS level (High PS) targeted a VT of 8–12 ml/kg. Two expiratory trigger (ET) levels were also sequentially applied in random order: a high ET level (High ET) set at 30 % of the peak inspiratory flow [50 % in chronic obstructive pulmonary disease (COPD) patients] and a low ET level (Low ET) set at 5 % of the peak inspiratory flow (30 % in COPD patients). Four distinct conditions were thus defined (High PS–Low ET, Low PS–Low ET, High PS–High ET and Low PS–High ET).
Measurements
Flow and pressure
Flow was measured with a heated Fleisch pneumotachograph (Hans Rudolph, Kansas City, MO, USA) and airway pressure was measured by a pressure transducer (DP 15–32, Validyne, Northridge, CA, USA).
Electromyography
The EMG signals were collected using surface electrodes (Kendall, Tyco Healthcare, Germany). Bilateral parasternal intercostal-target recordings were obtained from the second intercostal space, close to the sternum (Fig. 2). Bilateral scalene-targeted recordings were obtained in the posterior triangle of the neck at the level of the cricoid cartilage. Alae nasi-targeted recordings were obtained by placing one electrode on each nostril (Fig. 2).
Schematic representation of surface electrodes placement to record electromyographic activity (EMG) of parasternals (upper panel), scalene and Alae Nasi muscles (lower panel) Bilateral parasternal intercostal-target recordings were obtained from the second intercostal space, close to the sternum. Bilateral scalene-targeted recordings were obtained in the posterior triangle of the neck between the sternocleidomastoid muscle and the clavicle. The best side was retained for iEMG analyses. Alae nasi-targeted recordings were obtained by placing one electrode on each nostril
Dyspnea and comfort
At the end of each study period, dyspnea was rated using a visual analog scale (VAS) [8–10], and respiratory comfort was evaluated using the Adaptation to the Intensive Care Environment scale (ATICE) [7].
Data analysis
EMG signals were averaged according to Hug et al. [11] (see Figure E1 in the ESM). This produced a mean iEMG-RMS signal that was used to measure the maximum iEMG activity (EMGmax) and the iEMG area under the curve (EMGAUC), expressed as the percentages of their maximum value. The ratio of EMGAUC to VT was calculated as an index of neuro-mechanical coupling (EMGAUC/VT).
Ineffective efforts were defined as an abrupt airway pressure drop (≥0.5 cmH2O) simultaneous to a flow decrease (in absolute value) and a concomitant EMG activity on extra diaphragmatic signals not followed by an assisted cycle during the expiratory period [11, 12].
Statistical analysis
The statistical analysis was performed using the Prism 4.01 software (GraphPad Software, San Diego, CA). Normality testing (Kolmogorov–Smirnov) consistently failed: results are, therefore, expressed as median (25–75 interquartile range) and non-parametric statistical tests were used. A Friedman analysis of variance was performed to compare the four ventilatory assistance conditions in terms of EMGmax, EMGAUC and EMGmin, followed, when appropriate, by a pairwise comparison using Dunn’s post hoc test. Comparison between the EMID of the three muscles were performed using a Kruskal–Wallis test. The relationship between dyspnea and iEMG values, VT or ineffective efforts, was examined using the Spearman’s correlation. The normalisation/denormalisation technique described by Poon [13] was used to account for the use of within-patients replications in the correlation calculations. Differences were considered significant when the probability p of a type I error was below 5 %.
Results
Breathing pattern
Tidal volume was within the targeted ranges. Low PS VTs were significantly lower than High PS VTs (p < 0.001, Fig. 3, see also Table E1 in the ESM). Respiratory rates were generally higher under Low PS than under High PS, but the difference only reached statistical significance between Low PS–High ET and High PS–Low ET (p < 0.05, Fig. 3, see also Table E1 in the ESM). Similarly, SpO2 was constant without PEEP or FiO2 changes during the four conditions.
Respiratory rate, tidal volume, dyspnea intensity as assessed with the visual analogic scale (VAS) and adaptation to the Intensive Care Environment scale (ATICE) High PS high pressure support level targeting a tidal volume of 8–12 ml/kg, Low PS low pressure support level targeting a tidal volume of 6–8 ml/kg, High-ET high expiratory trigger level (30 % of the peak inspiratory flow, 50 % in COPD patients); Low ET low expiratory trigger level (5 % of the peak inspiratory flow, 30 % in COPD patients). Columns are median and bars are interquartile range. * p < 0.05 with Low PS–High ET; $ p < 0.05 with Low PS–Low ET
EMG activity
Parasternal intercostal and Alae Nasi EMGs were successfully recorded in all of the patients. A scalene EMG could not be recorded in 1 case (patient # 2). Overall, EMGmax and EMGAUC tended to be greater under Low PS than under High PS, whichever the muscle considered (Fig. 4, see also Table E2 in the ESM). This difference was consistently significant between Low PS and High PS–Low ET). The impact of the ET level on EMG activity was less marked than the impact of the PS level: for a given level of PS (Low PS or High PS), changes in ET level did not have a significant impact on EMGmax nor EMGAUC. The electromechanical inspiratory delay from iEMG onset to flow onset was significantly longer for the Alae Nasi [0.40 (0.20−0.61) s] than for the scalene and the parasternal intercostals [0.22 (0.00–0.31) and 0.21 (0.05–0.30) s, respectively; p < 0.0001]. They were not affected by ventilator settings.
Electromyographic (EMG) activity of scalene, intercostal parasternal and Alae nasi muscles Peak of the averaged electromyographic activity (EMG max , Panel A) and area under the averaged EMG activity (EMG AUC , Panel B). EMGmax and EMGAUC are expressed as a proportion of the maximum value. High PS high pressure support level targeting a tidal volume of 8–12 ml/kg; Low PS low pressure support level targeting a tidal volume of 6–8 ml/kg; High-ET, high expiratory trigger level (30 % of the peak inspiratory flow, 50 % in COPD patients); Low ET low expiratory trigger level (5 % of the peak inspiratory flow, 30 % in COPD patients). Columns are median. * p < 0.05 with Low PS–High ET; $ p < 0.05 with Low PS–Low ET
Neuro-mechanical coupling
In line with the above, scalene EMGAUC/VT and Alae Nasi EMGAUC/VT were significantly lower under the High PS–Low ET condition than under the Low PS regimen (p < 0.05). Similarly, parasternal EMGAUC/VT under High PS–Low ET was lower than under the Low PS–High ET condition [0.015 (0.000−0.049) and 0.198 (0.156–0.276), respectively; p < 0.001).
Ineffective triggering efforts
Ineffective triggering efforts were only observed in the five COPD patients (patients # 1 to # 4 and # 9). Their prevalence was higher with High PS [respectively, 1.8 (0.0–2.4) and 2.1 (1.8–2.2) min−1 with Low ET and High ET] than with Low PS conditions [respectively, 0.0 (0.0−0.3) and 0.0 (0.0−1.3) min−1 with Low ET and High ET; p < 0.05).
Dyspnea and comfort
Dyspnea ratings were significantly higher (p < 0.001) under Low PS, regardless of the ET level (Fig. 3). The ATICE score tended to be lower under Low PS than under High PS, but the difference reached statistical significance only between High PS–High ET and Low PS–High ET (p < 0.001).
Dyspnea ratings were negatively correlated with VT (ρ = −0.45, 95 % confidence interval (CI) 0.66 to −0.16, p = 0.003) and positively correlated with EMGmax [Alae nasi: ρ = 0.90 (0.81−0.95), p < 0.0001; scalene: ρ = 0.96 (0.91−0.98), p < 0.0001; parasternal intercostal: ρ = 0.90 (0.81−0.95), p < 0.0001] and EMGAUC [Alae nasi: ρ = 0.86 (0.74−0.92), p < 0.0001; scalene: ρ = 0.92 (0.83–0.96), p < 0.0001; parasternal intercostal ρ = 0.93 (0.87−0.96), p < 0.0001]. Positive correlations were also found, expectedly, between dyspnea ratings and EMGAUC/VT (Alae nasi: ρ = 0.79 (0.62−0.89); p < 0.0001; scalene: ρ = 0.89 (0.78−0.94); p < 0.0001; parasternal intercostal: ρ = 0.89 (0.80−0.94); p < 0.0001). Of note, an inverse correlation was found between dyspnea and ineffective triggering efforts [ρ= −0.85, (−0.96 to −0.50), p < 0.001]. The ATICE score was positively correlated with the tidal volume score [ρ = 0.79 (0.63−0.88), p < 0.0001].
Discussion
This study shows that the EMG activity of extra diaphragmatic inspiratory muscle responds to loading/unloading in acutely ill, mechanically ventilated patients. The study also evidences a strong relationship between the activity of these muscles and the intensity of dyspnea.
Inspiratory EMGs and ventilatory support
We used an inspiratory phase-locked EMG analysis technique to optimise inspiratory neck muscles surface recordings [11] and to study the responsiveness of extra diaphragmatic inspiratory muscles to inspiratory loading/unloading. In ICU patients, increased inspiratory neck muscles activity has been associated with insufficient levels of IPS during ventilator weaning [14], ventilator trigger asynchrony [15], and weaning failure [16]. Recent data indicate that the EMG activity of the parasternal intercostals, closely related to inspiratory drive, could provide a clinically useful biomarker in evaluating treatment response during acute exacerbations of COPD [17]. In this context, our study extends the current knowledge by showing that both an upper airway dilator (Alae nasi), and neck and rib cage inspiratory muscles similarly respond to the level of ventilatory support provided to mechanically ventilated ICU patients.
Of note, this study is seemingly the first to document a load-related activity of the Alae nasi in adult patients placed under mechanical ventilation. This is of importance, given the easy accessibility of this muscle for monitoring purposes and the easiness to obtain cross-talk free quality recordings. In our patients, Alae nasi was activated significantly before the scalenes and the parasternal intercostals, as physiologically expected [18], and in spite of the upper airway being bypassed by the endotracheal prosthesis.
Dyspnea and inspiratory EMG
In our patients, the intensity of dyspnea was closely correlated with the various EMG indices of extra diaphragmatic inspiratory muscles activity. Two particular features warrant emphasis. Firstly, the dyspnea-EMG relationships were particularly strong in our patients, with circa two-thirds of the variance of dyspnea being explained by EMG values. This relationship is far stronger than that which has previously been described in normal subjects with exactly the same methodology [19]. One possible explanation for this observation could be the tightness of the neuro-mechanical coupling in ICU patients as compared to normal individuals [20].
Secondly, in those of our patients diagnosed with COPD, dyspnea was the less intense under the highest levels of assistance (Fig. 3). Yet this corresponded to the highest prevalence of patient–ventilator asynchronies of the “ineffective triggering effort” type (inclusive of, in line with a simple cross correlation phenomenon, an inverse relationship between dyspnea and the prevalence of ineffective efforts). This situation is characteristic of dynamic hyperinflation [4, 15, 21]. Lower levels of ventilatory support in our patients dramatically decreased the prevalence of ineffective efforts, but were accompanied by an increase of the intensity of dyspnea and EMG activity. This is a typical example of “Gordian knot” situations where a ventilatory management strategy that is aimed at reducing morbidity exposes the patients to dyspnogenic stimuli. Indeed, patient–ventilator asynchronies are associated with negative clinical outcomes such as an increased duration of an ICU stay and a more frequent recourse to tracheostomy [4]. Intensivists are generally keen to avoid them. However, fighting ineffective triggering asynchronies by reducing the level of assistance induces dyspnea. Yet dyspnea is a noxious sensation [22] that should absolutely be avoided, mostly for obvious reasons of comfort, but also because of its possible association with negative clinical outcomes [1]. This concern could become increasingly prominent with the generalization of low tidal volume ventilation strategies beyond ARDS [23, 24].
Study limitations
Our population is a convenience sample of limited size. Although we were able to evidence statistically significant results, this sets an obvious limit to our results regarding generalisability. Our results, however, extend to actual ICU patients the proof of concept data already available in normal subjects [19]. Also, we studied surface EMGs (as opposed to needle EMGs) for the sake of simplicity, comfort and safety. Except for the alae nasi, this implies that we cannot be entirely be precise regarding the muscles sampled because of cross-talk issues. This should, however, not change the messages very much. Finally, we did not perform any measure of diaphragm function, which prevents us from providing a full description of respiratory muscle recruitment in our patients. This was not the purpose of the study; rather, it was designed with practical applications in mind. In addition, there are reasons to think that even if an increased diaphragm activity is related to breathlessness in certain situations, diaphragm activity may not be the most pertinent index to monitor in situations of acute inspiratory loading [25] where extra diaphragmatic inspiratory muscles become the prominent actors of the act of breathing.
Conclusions and perspective
In a recent study, 47 % of patients receiving mechanical ventilation reported being dyspneic on the first day when they were able to answer questions from the investigator. This is a very high figure, all the more so that the median rating of the dyspneic sensation on a visual analogue scale was slightly above 5. In 35 % of these patients, dyspnea receded after elementary ventilator settings adjustments [1], or, in other words, after improving the load-capacity balance of the system through external unloading. Dyspnea was also associated with anxiety and a longer duration of the ICU stay. These observations, beyond mere common sense, emphasize the importance of detecting and monitoring dyspnea in the ICU, with the aim of alleviating it. Particularly (but not solely) in patients with an impaired verbal communication, surrogates to words are needed. We submit that the results of the present study justify further efforts to validate surface EMGs of extra diaphragmatic inspiratory muscles as such surrogates. They would have the advantage of providing continuous monitoring to alert caregivers about putative dyspnea and prompt dyspnea targeted evaluations that would start, whenever possible, by asking the patient about his or her respiratory sensations. They would also help to titrate ventilatory assistance to patients’ needs. This titration could be achieved by analyzing extra diaphragmatic inspiratory muscle EMG as a surrogate biomarker of dyspnea and an indicator of probable under assistance on one hand, and by identifying ineffective triggering (from pressure and flow signals) as a marker of over assistance on the other hand. Practically, a reduction of the EMG activity of extra diaphragmatic inspiratory muscles after increasing the level of ventilator assistance with no or few ineffective triggering might suggest that ventilator support is well-tailored.
References
Schmidt M, Demoule A, Polito A, Porchet Rl, Aboab J, Siami S, Morelot-Panzini C, Similowski T, Sharshar T (2011) Dyspnea in mechanically ventilated critically ill patients. Crit Care Med 39:2059–2065
Vitacca M, Bianchi L, Zanotti E, Vianello A, Barbano L, Porta R, Clini E (2004) Assessment of physiologic variables and subjective comfort under different levels of pressure support ventilation. Chest 126:851–859
Thille AW, Cabello B, Galia F, Lyazidi A, Brochard L (2008) Reduction of patient-ventilator asynchrony by reducing tidal volume during pressure-support ventilation. Intensive Care Med 34:1477–1486
Thille AW, Rodriguez P, Cabello B, Lellouche Fo, Brochard L (2006) Patient-ventilator asynchrony during assisted mechanical ventilation. Intensive Care Med 32:1515–1522
Schmidt M, Chiti L, Hug F, Demoule A, Similowski T (2011) Surface electromyogram of inspiratory muscles: a possible routine monitoring tool in the intensive care unit. Br J Anaesth 106:913–914
Ramsay MA, Savege TM, Simpson BR, Goodwin R (1974) Controlled sedation with alphaxalone–alphadolone. Br Med J 2:656–659
De Jonghe B, Cook D, Griffith L, Appere-de-Vecchi C, Guyatt G, Theron V, Vagnerre A, Outin H (2003) Adaptation to the Intensive Care Environment (ATICE): development and validation of a new sedation assessment instrument. Crit Care Med 31:2344–2354
Lush MT, Janson-Bjerklie S, Carrieri VK, Lovejoy N (1988) Dyspnea in the ventilator-assisted patient. J Crit Care 17:528–535
Bouley GH, Froman R, Shah H (1992) The experience of dyspnea during weaning. J Crit Care 21:471–476
Powers J, Bennett SJ (1999) Measurement of dyspnea in patients treated with mechanical ventilation. Am J Crit Care 8:254–261
Hug F, Raux M, Prella M, Morelot-Panzini C, Straus C, Similowski T (2006) Optimized analysis of surface electromyograms of the scalenes during quiet breathing in humans. Respir Physiol Neurobiol 150:75–81
Georgopoulos D, Prinianakis G, Kondili E (2006) Bedside waveforms interpretation as a tool to identify patient-ventilator asynchronies. Intensive Care Med 32:34–47
Poon CS (1988) Analysis of linear and mildly nonlinear relationships using pooled subject data. J Appl Physiol (Bethesda, Md: 1985) 64:854–859
Brochard L, Harf A, Lorino H, Lemaire F (1989) Inspiratory pressure support prevents diaphragmatic fatigue during weaning from mechanical ventilation. Am Rev Respir Dis 139:513–521
Chao DC, Scheinhorn DJ, Stearn-Hassenpflug M (1997) Patient-ventilator trigger asynchrony in prolonged mechanical ventilation. Chest 112:1592
Parthasarathy S, Jubran A, Laghi F, Tobin MJ (2007) Sternomastoid, rib cage, and expiratory muscle activity during weaning failure. J Appl Physiol (Bethesda, Md: 1985) 103:140–147
Murphy PB, Kumar A, Reilly C, Jolley C, Walterspacher S, Fedele F, Hopkinson NS, Man WD-C, Polkey MI, Moxham J, Hart N (2011) Neural respiratory drive as a physiological biomarker to monitor change during acute exacerbations of COPD. Thorax 66:602–608
Strohl KP, Hensley MJ, Hallett M, Saunders NA, Ingram RH Jr (1980) Activation of upper airway muscles before onset of inspiration in normal humans. J Appl Physiol 49:638–642
Chiti L, Biondi G, Morelot-Panzini C, Raux M, Similowski T, Hug FO (2008) Scalene muscle activity during progressive inspiratory loading under pressure support ventilation in normal humans. Respir Physiol Neurobiol 164:441–448
Zakynthinos SG, Vassilakopoulos T, Roussos C (1995) The load of inspiratory muscles in patients needing mechanical ventilation. Am J Respir Crit Care Med 152:1248–1255
Leung P, Jubran A, Tobin MJ (1997) Comparison of assisted ventilator modes on triggering, patient effort, and dyspnea. Am J Respir Crit Care Med 155:1940–1948
Morelot-Panzini C, Demoule A, Straus C, Zelter M, Derenne J-P, Willer J-C, Similowski T (2007) Dyspnea as a noxious sensation: inspiratory threshold loading may trigger diffuse noxious inhibitory controls in humans. J Neurophysiol 97:1396–1404
Lellouche F, Lipes J (2012) Prophylactic protective ventilation: lower tidal volumes for all critically ill patients? Intensive Care Med 39:6–15
Serpa Neto A, Cardoso SO, Manetta JA, Pereira VG, Esposito DC, Pasqualucci Mde O, Damasceno MC, Schultz MJ (2012) Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta-analysis. JAMA 308:1651–1659
Aliverti A, Cala SJ, Duranti R, Ferrigno G, Kenyon CM, Pedotti A, Scano G, Sliwinski P, Macklem PT, Yan S (1997) Human respiratory muscle actions and control during exercise. J Appl Physiol (Bethesda, Md: 1985) 83:1256–1269
Acknowledgments
We thank Mr. Paul E. Robinson for reviewing the manuscript. Matthieu Schmidt was supported by ANTADIR (Association Nationale pour le Traitement à Domicile, l’Innovation et la Recherche) and the Fonds d’etude et de recherche du corps medicale des Hôpitaux de Paris, France. Alexandre Demoule was supported by CARDIF (Centre d’Assistance Respiratoire à Domicile d’Ile-de-France), the Société de Réanimation Langue Française and the Société de Pneumologie de Langue Française, Paris, France. François Hug was supported by CARDIF (Centre d’Assistance Respiratoire à Domicile d’Ile-de-France), Paris, France. The study was funded by Association pour le Développement et l’Organisation de la Recherche en Pneumologie et sur le Sommeil (ADOREPS), Paris, France, a by the grant “EEG-PVI “ (ANR-11-EMMA-030-01) of Agence Nationale de la Recherche, Paris, France
Conflicts of interest
In 2009 and 2010, the Association pour le Développement et l’Organisation de la Recherche en Pneumologie et sur le Sommeil (ADOREPS) received an unrestricted research grant from Maquet France SA, Orléans, France, to support pathophysiological research studies on the “neurally adjusted ventilatory assist” (NAVA) mode.
Author information
Authors and Affiliations
Corresponding author
Additional information
T. Similowski and A. Demoule contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Schmidt, M., Kindler, F., Gottfried, S.B. et al. Dyspnea and surface inspiratory electromyograms in mechanically ventilated patients. Intensive Care Med 39, 1368–1376 (2013). https://doi.org/10.1007/s00134-013-2910-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-013-2910-3