Abstract
Objectives
To compare the design and results of randomized trials investigating prolonged glucocorticoid treatment (≥ 7 days) in patients with acute lung injury–acute respiratory distress syndrome (ALI–ARDS), and review factors affecting response to therapy, including the role of secondary prevention.
Design
Trials were retrieved from the Cochrane Central Register of Controlled Trials (CENTRAL). Two investigators collected data on study characteristics, treatment intervention, and outcomes. The methodological quality of trials was determined and data were analyzed with Review Manager 4.2.3.
Measurements and results
Five selected trials (n = 518) consistently reported significant improvement in gas exchange, reduction in markers of inflammation, and decreased duration of mechanical ventilation and intensive care unit stay (all p < 0.05). Two early small clinical trials showed marked reductions in the relative risk (RR) of death with glucocorticoid therapy (RR = 0.14, 95% CI 0.04–0.53; p = 0.004, I2 = 0%). Three subsequent larger trials, when combined, although nominally beneficial, did not reproduce the marked reductions observed in the earlier trials (RR = 0.84; 95% CI 0.68–1.03; p = 0.09, I2 = 9.1%), but achieved a distinct reduction in the RR of death in the larger subgroup of patients (n = 400) treated before day 14 of ARDS [82/214 (38%) vs. 98/186 (52.5%), RR = 0.78; 95% CI 0.64–0.96; p = 0.02, I2 = 0%].
Conclusions
Prolonged glucocorticoid treatment substantially and significantly improves meaningful patient-centered outcome variables, and has a distinct survival benefit when initiated before day 14 of ARDS.
Similar content being viewed by others
Introduction
In acute respiratory distress syndrome (ARDS), the evolution of systemic and pulmonary inflammation in the first week of mechanical ventilation determines the physiological progression (resolving vs. unresolving) and outcome of the disease [1–3]. Patients failing to improve the lung injury score (LIS) or its components by day 7 of ARDS (unresolving ARDS), contrary to those who are improving, have persistent elevation in circulating and bronchoalveolar lavage (BAL) levels of inflammatory cytokines and chemokines, markers of alveolocapillary membrane permeability and fibrogenesis (dysregulated systemic inflammation) [1–4], and a higher mortality [5–7].
Translational research has provided evidence that insufficient glucocorticoid receptor (GR)-mediated inhibition of proinflammatory transcription factor nuclear factor-κB (NF-κB) is a central pathogenetic mechanism of dysregulated systemic and pulmonary inflammation in ARDS and is potentially reversed by quantitatively and temporally adequate prolonged glucocorticoid administration [1, 2, 8]. In a small randomized trial, prolonged methylprednisolone (2 mg/kg/day) administration led to rapid, progressive, and sustained reductions in plasma and BAL inflammatory cytokines, chemokines, and procollagen levels with parallel improvement in LIS and multiple organ dysfunction syndrome score (MODS) [2, 8–10] Treatment was associated with significant reductions in duration of mechanical ventilation and in intensive care unit (ICU) mortality [9]. Despite its limitations [11], this trial provided proof of concept for methylprednisolone treatment in ARDS [4, 8, 10], justifying a larger confirmatory trial that was conducted over 6 years by the ARDS Clinical Trials Network [12]. During this period, three additional randomized trials were conducted (Table 1) to investigate prolonged glucocorticoid treatment in early acute lung injury (ALI) (PaO2:FiO2 < 300) [13] and ARDS (PaO2:FiO2 < 200) [14, 15]. Cumulative data from these trials involving a total of 518 patients provide a broader understanding of the effects of glucocorticoid treatment in ALI–ARDS.
The purpose of this commentary is to highlight differences in study design between the two unresolving ARDS trials [10, 12] (Table 2) and to evaluate critically the findings of the ARDS Network trial [12]. This analysis considers previously reviewed factors affecting response to prolonged glucocorticoid administration (Fig. 1) [16] and the results of the other randomized trials [9, 13–15].
Methods for data extraction and analysis
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), published in The Cochrane Library (issue 2, 2007), using the search terms “acute lung injury,” “acute respiratory distress syndrome,” “acute respiratory failure,” “steroids,” “adrenal cortex hormones,” “glucocorticoids,” and “corticosteroids.” We also searched the following electronic databases using the topic search terms in combination with a search strategy for identifying trials developed by The Cochrane Collaboration: (1) MEDLINE (1966 to May 2007) using the same search terms.
Data were extracted by one author (G.U.M.) and checked by another author (D.A.). Then they were entered onto the Review Manager 4.2.3 version by one author using the double-entry option (D.A.) and checked by another author (G.U.M.). For each outcome measure, we computed 2 × 2 tables summarizing the number of people who experienced the event or outcome in each comparison group and the total number in each group. These tables were organized so that a beneficial effect of treatment was associated with a relative risk (RR) < 1. We performed intention-to-treat analyses. We performed all statistical calculations using Review Manager 4.2.3. We calculated a weighted treatment effect (using the fixed effect model) across trials. The results were expressed as RRs with 95% confidence intervals (CI) for dichotomous outcomes (i. e., mortality), and weighted mean difference (WMD, 95% CI) for continuous outcomes (i. e., mechanical ventilation-free days). We considered methods based on the random effects model only in the case of heterogeneity (i. e., 10% level of statistical significance for the chi-squared test for homogeneity). To identify potential sources of heterogeneity (when the chi-squared test for homogeneity yielded a probability value < 0.10), we sought to conduct a subgroup analysis based on size of the study (small vs. larger), timing of initiation of treatment (before day 14 from the onset of ALI–ARDS), and duration of treatment (greater than 1 week).
Findings during treatment
Table 3 shows the results of the ARDS network trial during treatment. In agreement with all other trials, prolonged glucocorticoid treatment was associated with significant improvement in PaO2:FiO2 ratio [9, 13–15] and significant reduction in markers of systemic inflammation [8, 13–15], BAL neutrophilia [4], duration of mechanical ventilation [9, 13, 15], and ICU stay [9, 13, 15]. In the five trials [9, 12–15], glucocorticoid treatment increased the number of mechanical ventilation-free days at day 28 (4.42 days; 95% CI 2.93–5.90; p < 0.001). However, there was significant heterogeneity across the studies (Chi2 = 15.36, p = 0.004). Subgroup analysis based on studies that investigated only treatment (methylprednisolone) of greater than 1 week's duration (n = 295) [9, 13, 15] showed a distinct increase in the number of mechanical ventilation-free days (WMD = 5.59 days, 95% CI 3.49–7.68; p < 0.001) without heterogeneity (Chi2 = 2.18, p = 0.34) across the studies (Fig. 2). This effect was far greater than the one observed with the recommended low tidal volume ventilation (12 ± 11 vs. 10 ± 11; p = 0.007) [17] or conservative strategy of fluid management (14.6 ± 0.5 vs. 12.1 ± 0.5; p < 0.001) [18].
Findings after rapid tapering of treatment
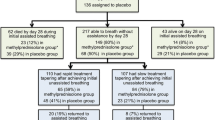
In the ARDS network trial, the large benefits observed during methylprednisolone treatment of unresolving ARDS (10 days' reduction in duration of mechanical ventilation; 27% relative reduction in mortality; 21% relative increase in patients discharged home after initial weaning) were partially lost with protocol-driven rapid tapering of the study drug after 48 h of unassisted breathing (Table 2). It is important to underscore that, in ARDS, systemic and pulmonary inflammation continue for weeks and extend well beyond extubation [8]. The circulating half-life of methylprednisolone varies from 3.8 to 7.2 h, with greatly diminished effects expected 24–36 h after discontinuing treatment [19–21]. Moreover, exposure to glucocorticoids leads to significant reduction in glucocorticoid receptor number and binding capacity (reviewed in [2]). Ample evidence demonstrates that rapid tapering of glucocorticoid treatment may lead to rebound inflammation–fibroproliferation [10, 20, 22–27] and an exaggerated cytokine response to infection [28]. In experimental ALI, prolonged glucocorticoid administration decreased edema and lung collagen formation, while early withdrawal rapidly negated the positive effects of therapy [22–24]. In unresolving ARDS, early discontinuation of methylprednisolone administration was associated with physiological deterioration [10, 25, 26] that improved following reinstitution of treatment [25, 26]. Likewise, in patients with septic shock, early glucocorticoid discontinuation was associated with physiological deterioration [20, 27]. In the ARDS network trial, methylprednisolone was removed within 3–4 days of extubation and likely contributed, as acknowledged by the authors, to the deterioration in PaO2:FiO2 ratio and higher rate of reintubation (35% with shock and probable treatment-induced relative adrenal insufficiency) and associated mortality (Table 3) [12]. In other trials [9, 13–15], longer duration of methylprednisolone treatment following extubation – similar to the treatment of respiratory failure associated with asthma or chronic obstructive lung disease (COPD) – was not associated with a relapse of ARDS.
Findings for patients randomized after day 14
Although the ARDS network trial reported substantial and significant positive results for most secondary variables (Table 3), the trial is portrayed as negative partly because of the increased mortality observed in the subgroup of patients randomized to methylprednisolone after day 14 of ARDS. This small subgroup, however, had large imbalances in baseline characteristics (Table 4) for age, gender, pneumonia, trauma, serum creatinine, APACHE III, compliance, and lung injury score that likely accounted for the uncharacteristically low mortality in the control group (8% vs. 36%). Prior studies have shown that risk factors for higher mortality in ARDS include age [29–31], female gender [32], sepsis [29], APACHE score [30, 31, 33, 34], and lung injury score [33], while trauma is associated with lower mortality [29, 35]. As shown in Table 4, the control group had – among all four subgroups – the lowest values for age, pneumonia, APACHE III, creatinine, and lung injury score and the highest values for trauma and compliance. In contrast, the treated group had – among all subgroups – the highest values for age, female gender, pneumonia, and lung injury score and the lowest value for compliance. These imbalances and the small size of the subgroup directly challenge the conclusion of the ARDS network trial that “starting methylprednisolone therapy more than two weeks after the onset of ARDS may increase the risk of death” [12]. When mortality was adjusted (Table 4) fitting in the regression model, some variables found different at baseline [APACHE III, age, plateau pressure, number of organ failures, alveolar–arterial O2 difference (A-a DO2)], the 60-day mortality decreased to 11.2 ± 7.2% vs. 28.0 ± 7.5%, and significance (p = 0.57) was lost (personal communication: Dr. Marek Ancukiewicz, Massachusetts General Hospital, Boston, MA, USA).
Preventive measures to decrease complications associated with glucocorticoid treatment
As shown in Fig. 1, implementing measures to prevent complications associated with glucocorticoid treatment affects overall response and is fundamental to minimizing imbalances in risk exposure between groups. Failed or delayed recognition of nosocomial infections in the presence of a blunted febrile response represents a serious threat to the recovery of patients receiving prolonged glucocorticoid treatment [16]. In a randomized trial, therefore, inclusion of infection surveillance – for those receiving treatment greater than 7 days' duration – is essential to minimize the potential bias generated by under-diagnosed infections on morbidity and mortality. In the two randomized trials [9, 15] that incorporated infection surveillance (Table 1), nosocomial infections were frequently (56%) identified in the absence of fever. After randomizing patients to methylprednisolone, researchers performed 79 bronchoscopies with bilateral BAL every 5–7 days and identified 19 cases of ventilator-associated pneumonia, 9 in afebrile patients [9, 15]. Although the ARDS Network trial reported a lower rate of clinically identified ventilator-associated pneumonia in treated patients; the protocol did not incorporate infection surveillance, making it impossible to estimate the impact of undiagnosed infections on outcome.
The combination of glucocorticoids and neuromuscular blocking agents versus steroids alone significantly increases the risk for prolonged neuromuscular weakness [36]. For this reason, the use of neuromuscular blocking agents is strongly discouraged in patients receiving concomitant glucocorticoid treatment, particularly when other risk factors are present (sepsis, aminoglycosides, etc.). In the ARDS Network trial, although all groups had similar exposure to paralytic agents (49% vs. 42%; p = 0.30), those randomized to methylprednisolone had a higher rate of serious events associated with myopathy or neuropathy (Table 3) [12]. ICU-acquired paresis is a known independent predictor of prolonged weaning [37]. However, among the 43 patients with weakness, those randomized to methylprednisolone (n = 25) had a significant (p = 0.003) and sizable (11 days) reduction in duration of mechanical ventilation (Table 7, supplementary appendix) [12].
Randomization of patients with undetectable procollagen in BAL
Persistent ARDS is characterized by ongoing inflammation, parenchymal cell proliferation, and disordered deposition of collagen, all of which may be responsive to glucocorticoid therapy [12]. Yet, in the ARDS Network trial, procollagen – a marker of fibrogenesis – was undetectable in 40% of BAL samples. In the previous late ARDS study [10], all patients with unresolving ARDS had a progressive increase in plasma and BAL procollagen type I and III until randomization to methylprednisolone. Lack of procollagen in BAL is more indicative of resolved ARDS, a condition that would not benefit from late introduction of anti-inflammatory anti-fibrotic treatment. This might explain why, in the ARDS network trial [12], those with no or low BAL procollagen levels in contrast to those with elevated procollagen levels had a poor response to methylprednisolone treatment.
Effect of prolonged glucocorticoid treatment on mortality
The analysis for mortality is limited by the significant heterogeneity across the five trials (Chi2 = 9.22, p = 0.06). In these trials, glucocorticoid treatment reduced short-term mortality in models with fixed effects [91/276 (33%) vs. 111/242 (46%), RR = 0.76, 95% CI 0.62–0.93; p = 0.007]. Subgroup analysis for size of the study (Fig. 3) showed little heterogeneity within the two small studies [9, 13] and within the three larger trials [12, 14, 15]. The two early small clinical trials (n = 68) [9, 13] showed marked reductions in RR of death with glucocorticoid therapy [2/39 (5%) vs. 11/31 (35%), RR = 0.14, 95% CI 0.04–0.53; p = 0.004, I2 = 0%]. The three subsequently published larger clinical trials, when combined (n = 448) [12, 14, 15], although nominally beneficial, did not reproduce the marked reductions observed in the earlier trials [89/237 (37.5%) vs. 1000/211 (47%), RR = 0.84; 95% CI 0.68–1.03; p = 0.09, I2 = 9.1%], but achieved a distinct reduction in the RR of death in the larger subgroup (n = 400) of patients treated before day 14 of ARDS [82/214 (38%) vs. 98/186 (52.5%), RR = 0.78; 95% CI 0.64–0.96; p = 0.02, I2 = 0%]. We interpret these data to indicate that treatment initiation before day 14 of ALI–ARDS has a beneficial effect on mortality. This is supported by the fact that when analyzing the three trials investigating methylprednisolone for greater than 1 week's treatment (the form of treatment most likely to be used in ARDS) initiated before day 14 of ARDS (n = 245), mortality was equally decreased [35/144 (24%) vs. 40/101 (40%), RR = 0.62, 95% CI 0.43–0.90; p = 0.01, I2 = 17.7%] [38].
Effects of prolonged glucocorticoid treatment on ARDS survival. The top panel reports data for all five randomized trials. The middle panel reports data for all five randomized trials after removing patients randomized after day 14. The bottom panel reports data for the three randomized trials that investigate prolonged methylprednisolone treatment of greater than 1 week's duration after removing patients randomized after day 14. This figure is reproduced with permission from [38]. Mortality was the primary outcome in three of the five studies [9, 12, 14]. One of these studies [14] investigated the efficacy of low-dose hydrocortisone in septic shock patients with ARDS by post-hoc analysis of a previously completed trial. Mortality is reported as 28-day [14], hospital [9, 13, 15], or 60-day [12]
In conclusion, we have reviewed some of the salient differences in two trials investigating the effectiveness of prolonged methylprednisolone treatment in unresolving ARDS [9, 12]. Both trials [9, 12], similar to others [13, 15], reported a significant biological and physiological improvement during methylprednisolone treatment resulting in a significant reduction in duration of mechanical ventilation. None of the glucocorticoids trials reported an increased rate of infection, while two reported a reduction [12, 15]. The more rapid resolution of ALI–ARDS, observed in all five randomized trials, might have a positive impact on long-term physical function and survival [15, 39]. In the ARDS network trial, premature removal of an effective treatment likely accounted for the higher rate of reintubation and loss of early survival benefits [12]. Moreover, the conclusion that methylprednisolone treatment increases mortality in patients randomized after day 14 is challenged by the large imbalances in baseline characteristics in this small subgroup of patients and the loss of significance when mortality is adjusted for baseline differences. The number of patients (n = 518) recruited in the above-referenced randomized trials is similar to those included in randomized trials for status asthmaticus (n = 507), COPD (n = 511), or P. jiroveci pneumonia (n = 352) [40]. Taken together, the results of five randomized studies [9, 12–15] provide evidence of efficacy in ALI–ARDS (accelerated resolution of ARDS with significant reduction in duration of mechanical ventilation and ICU stay) with a favorable risk profile when secondary prevention is implemented. These measures include (1) intensive infection surveillance, (2) avoidance of paralytic agents, and (3) avoidance of rebound inflammation with premature discontinuation of treatment that may lead to physiological deterioration and reintubation.
Correct use of prolonged glucocorticoid treatment is associated with a substantial and significant improvement in meaningful patient-centered outcome variables, and a distinct survival benefit when treatment is initiated before day 14 of ARDS (Fig. 3). The findings recently reported with low-dose methylprednisolone (1 mg/kg/day) in early severe ARDS [15] should be replicated in a larger trial of patients with ALI–ARDS. The new trial should have mortality as the primary end-point, avoid internal crossover, and incorporate secondary prevention measures (infection surveillance and avoidance of paralysis and rebound inflammation) to minimize potential complications associated with glucocorticoid treatment. Similarly, clinicians should integrate secondary prevention measures when using prolonged glucocorticoid treatment in patients with ARDS. We hope that this commentary will generate interest on this important topic and stimulate additional clinical investigation of this inexpensive and highly effective anti-inflammatory therapy.
References
Meduri GU, Muthiah MP, Carratu P, Eltorky M, Chrousos GP (2005) Nuclear factor-kappaB- and glucocorticoid receptor alpha-mediated mechanisms in the regulation of systemic and pulmonary inflammation during sepsis and acute respiratory distress syndrome. Evidence for inflammation-induced target tissue resistance to glucocorticoids. Neuroimmunomodulation 12:321–338
Meduri GU, Yates CR (2004) Systemic inflammation-associated glucocorticoid resistance and outcome of ARDS. Ann N Y Acad Sci 1024:24–53
Parsons PE, Eisner MD, Thompson BT, Matthay MA, Ancukiewicz M, Bernard GR, Wheeler AP (2005) Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med 33:1–6
Sinclair S, Bijoy J, Golden E, Carratu P, Umberger R, Meduri GU (2006) Interleukin-8 and soluble intercellular adhesion molecule-1 during acute respiratory distress syndrome and in response to prolonged methylprednisolone treatment. Minerva Pneumol 45:93–104
Bone RC, Maunder R, Slotman G, Silverman H, Hyers TM, Kerstein MD, Ursprung JJ (1989) An early test of survival in patients with the adult respiratory distress syndrome. The PaO2/FiO2 ratio and its differential response to conventional therapy. Prostaglandin E1 Study Group. Chest 96:849–851
Bernard GR, Luce JM, Sprung CL, Rinaldo JE, Tate RM, Sibbald WJ, Kariman K, Higgins S, Bradley R, Metz CA (1987) High-dose corticosteroids in patients with the adult respiratory distress syndrome. N Engl J Med 317:1565–1570
Meduri GU (1997) Host defense response and outcome in ARDS. Chest 112:1154–1158
Meduri GU, Tolley EA, Chrousos GP, Stentz F (2002) Prolonged methylprednisolone treatment suppresses systemic inflammation in patients with unresolving acute respiratory distress syndrome. Evidence for inadequate endogenous glucocorticoid secretion and inflammation-induced immune cell resistance to glucocorticoids. Am J Respir Crit Care Med 165:983–991
Meduri GU, Headley S, Golden E, Carson S, Umberger R, Kelso T, Tolley E (1998) Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome. A randomized controlled trial. JAMA 280:159–165
Meduri GU, Tolley EA, Chinn A, Stentz F, Postlethwaite A (1998) Procollagen types I and III aminoterminal propeptide levels during acute respiratory distress syndrome and in response to methylprednisolone treatment. Am J Respir Crit Care Med 158:1432–1441
Wheeler A, Bernard GR, Schoenfeld D, Steinberg K (1998) Methylprednisolone for unresolving ARDS. JAMA 280:2074
Steinberg KP, Hudson LD, Goodman RB, Hough CL, Lanken PN, Hyzy R, Thompson BT, Ancukiewicz M (2006) Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med 354:1671–1684
Confalonieri M, Urbino R, Potena A, Piattella M, Parigi P, Puccio G, Della Porta R, Giorgio C, Blasi F, Umberger R, Meduri GU (2005) Hydrocortisone infusion for severe community-acquired pneumonia: a preliminary randomized study. Am J Respir Crit Care Med 171:242–248
Annane D, Sebille V, Bellissant E (2006) Effect of low doses of corticosteroids in septic shock patients with or without early acute respiratory distress syndrome. Crit Care Med 34:22–30
Meduri GU, Golden E, Freire AX, Taylor E , Zaman M , Carson SJ, Gibson M, Umberger R (2007) Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest 131:954–963
Meduri GU (1999) An historical review of glucocorticoid treatment in sepsis. Disease pathophysiology and the design of treatment investigation. Sepsis 3:21–38
Bower G, Matthay M (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 342:1301–1308
Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF Jr., Hite RD, Harabin AL (2006) Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 354:2564–2575
Rosenberg J, Lysz K (1977) An in vitro study of how much methylprednisolone is needed to produce immunosuppression. Proc Clin Dial Transplant Forum 7:23–28
Keh D BT, Weber-Cartens S, Schulz C, Ahlers O, Bercker S, Volk HD, Doecke WD, Falke KJ, Gerlach H (2003) Immunologic and hemodynamic effects of “low-dose” hydrocortisone in septic shock: a double-blind, randomized, placebo-controlled, crossover study. Am J Respir Crit Care Med 167:512–520
Yates CR, Vysokanov A, Mukherjee A, Ludden TM, Tolley EA, Meduri GU, Dalton JT (2001) Time-variant increase in methylprednisolone clearance in patients with acute respiratory distress syndrome: A population pharmocokinetic study. J Clin Pharmacol 41:1–10
Hesterberg TW, Last JA (1981) Ozone-induced acute pulmonary fibrosis in rats. Prevention of increased rates of collagen synthesis by methylprednisolone. Am Rev Respir Dis 123:47–52
Hakkinen PJ, Schmoyer RL, Witschi HP (1983) Potentiation of butylated-hydroxytoluene-induced acute lung damage by oxygen. Effects of prednisolone and indomethacin. Am Rev Respir Dis 128:648–651
Kehrer JP, Klein-Szanto AJ, Sorensen EM, Pearlman R, Rosner MH (1984) Enhanced acute lung damage following corticosteroid treatment. Am Rev Respir Dis 130:256–261
Ashbaugh DG, Maier RV (1985) Idiopathic pulmonary fibrosis in adult respiratory distress syndrome. Diagnosis and treatment. Arch Surg 120:530–535
Hooper RG, Kearl RA (1990) Established ARDS treated with a sustained course of adrenocortical steroids. Chest 97:138–143
Briegel J, Jochum M, Gippner-Steppert C, Thiel M (2001) Immunomodulation in septic shock: hydrocortisone differentially regulates cytokine responses. J Am Soc Nephrol 12(Suppl 17):S70–74
Barber AE, Coyle SM, Fischer E, Smith C, van der Poll T, Shires GT, Lowry SF (1995) Influence of hypercortisolemia on soluble tumor necrosis factor receptor II and interleukin-1 receptor antagonist responses to endotoxin in human beings. Surgery 118:406–410
Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD (2005) Incidence and outcomes of acute lung injury. N Engl J Med 353:1685–1693
Bollen CW, Uiterwaal CS, van Vught AJ (2006) Systematic review of determinants of mortality in high frequency oscillatory ventilation in acute respiratory distress syndrome. Crit Care 10:R34
Luecke T, Muench E, Roth H, Friess U, Paul T, Kleinhuber K, Quintel M (2006) Predictors of mortality in ARDS patients referred to a tertiary care centre: a pilot study. Eur J Anaesthesiol 23:403–410
Agarwal R, Aggarwal AN, Gupta D, Behera D, Jindal SK (2006) Etiology and outcomes of pulmonary and extrapulmonary acute lung injury/ARDS in a respiratory ICU in North India. Chest 130:724–729
Heffner JE, Brown LK, Barbieri CA, Harpel KS, DeLeo J (1995) Prospective validation of an acute respiratory distress syndrome predictive score. Am J Respir Crit Care Med 152(5 Pt 1):1518–1526
Roca O, Sacanell J, Laborda C, Perez M, Sabater J, Burgueno MJ, Dominguez L, Masclans JR (2006) Cohort study on incidence of ARDS in patients admitted to the ICU and prognostic factors of mortality. Med Intensiva 30:6–12
Salim A, Martin M, Constantinou C, Sangthong B, Brown C, Kasotakis G, Demetriades D, Belzberg H (2006) Acute respiratory distress syndrome in the trauma intensive care unit: morbid but not mortal. Arch Surg 141:655–658
Leatherman JW, Fluegel WL, David WS, Davies SF, Iber C (1996) Muscle weakness in mechanically ventilated patients with severe asthma. Am J Respir Crit Care Med 153:1686–1690
De Jonghe B, Bastuji-Garin S, Sharshar T, Outin H, Brochard L (2004) Does ICU-acquired paresis lengthen weaning from mechanical ventilation? Intensive Care Med 30:1117–1121
Meduri GU (2007) There is no illumination in speculation. Additional data in support of methylprednisolone treatment in ARDS. Chest 132:1097–1100
Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, Stewart TE, Barr A, Cook D, Slutsky AS (2003) One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med 348:683–693
Jantz MA, Sahn SA (1999) Corticosteroids in acute respiratory failure. Am J Respir Crit Care Med 160:1079–1100
Cronin L, Cook DJ, Carlet J, Heyland DK, King D, Lansang MA, Fisher CJ Jr (1995) Corticosteroid treatment for sepsis: a critical appraisal and meta-analysis of the literature. Crit Care Med 23:1430–1439
Annane D, Sebille V, Charpentier C, Bollaert PE, Francois B, Korach JM, Capellier G, Cohen Y, Azoulay E, Troche G, Chaumet-Riffaut P, Bellissant E (2002) Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA 288:862–871
Acknowledgements
We wish to acknowledge Dr. Peter Eichacker for critical review of the manuscript, Dr. David Armbruster for editorial assistance, and Dr. Marek Ancukiewicz (Massachusetts General Hospital, Boston) for providing previously unpublished data and statistical analysis for the ARDS Network.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meduri, G.U., Marik, P.E., Chrousos, G.P. et al. Steroid treatment in ARDS: a critical appraisal of the ARDS network trial and the recent literature. Intensive Care Med 34, 61–69 (2008). https://doi.org/10.1007/s00134-007-0933-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-007-0933-3